Abstract
Osteoporosis (OP) is a disease which causes bone loss and fractures, leading to severe pain and deformity. This study has aimed to assess the effects of pulsed wave low-level laser therapy (PW LLLT) on cortical bone in two experimental models of OP in rats. There were four ovariectomized (OVX-d) groups and four dexamethasone-treated groups. The healthy group were considered for baseline evaluations. At 14 weeks following ovariectomy, the OVX-d rats were further subdivided into the following: control rats with OP, OVX-d rats that received alendronate (1 mg/kg), OVX-d rats treated with LLLT, and OVX-d rats treated with alendronate and LLLT. The remaining rats received dexamethasone for 5 weeks and were divided into four groups: control, alendronate-treated rats (1 mg/kg), laser-treated rats, and laser-treated rats with concomitant administration of alendronate. The rats received alendronate for 30 days. LLLT (890 nm, 80 Hz, 0.972 J/cm2) was performed on the tibias three times per week for 8 weeks. After 8 weeks, tibias were extracted and submitted to a three-point bending test. PW LLLT did not increase the biomechanical parameters of osteoporotic bones compared to controls and healthy rats. PW LLLT associated with alendronate treatment significantly increased stress high load in OVX-d rats compared to the healthy group. PW LLLT at the current study parameters failed to cause beneficial biomechanical effects in the examined osteoporotic cortical bones. PW LLLT associated with alendronate treatment produced a more remarkable effect on bone strength in the ovariectomized induced OP rat model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis (OP) is a disease which causes bone loss and fractures and leads to severe pain, deformity, and in certain cases secondary complications that result in death [1]. In the USA, approximately 10 million adults older than 50 years are estimated to have OP, and another 34 million are at risk for this disease [2]. OP, in particular hip fractures, has a large economic impact; the direct costs of osteoporotic fractures in the USA in 2005 have been estimated at $19 billion [3]. The disease is classified clinically as either primary or secondary OP. Primary OP refers to bone loss that occurs both in postmenopausal women (type I) or is attributed to the normal aging process (type II). Secondary OP refers to bone loss that ensues as a secondary effect of other diseases or drug treatment. Postmenopausal OP (type I) is the most common form of this disease and is believed to initiate with deficiencies in estrogen production that follow menopause [4]. During estrogen deficiency, osteoclasts remove excess bone without adequate formation by osteoblasts. Bone loss ensues when the basic trabecular bone units (trabeculae) become thin and resorb completely or fracture [5]; ultimately, bone fractures occur under minimal trauma in the bones of the hips, wrists, and spine [5]. Although glucocorticoids (GCs) effectively suppress inflammation, their prolonged use is accompanied by bone loss that leads to OP. GC-induced OP (GIOP) is the most common cause of secondary OP [6].
Various animal models have been established and used to investigate the pathogenesis of OP as well as to facilitate preclinical testing and new treatment options such as antiresorptive drugs [7]. Histomorphometric parameters and biochemical markers of bone metabolism in animal studies only indicate a decrease in bone formation and minimal changes in bone resorption. These parameters are less important with regards to OP-associated fractures and investigations in orthopedic surgery. Furthermore, histological studies do not give direct information about the mechanical strength of the bone. The ultimate reason for a bone fracture following minimal trauma is a reduction in mechanical strength [8]. On the other hand, bone densitometry is often used as a surrogate to evaluate bone fragility, but direct biomechanical testing of the bone undoubtedly provides more information about mechanical integrity [9].
There is an increasing need for strategies that prevent bone loss. Numerous treatments are planned and include estrogen therapy, bisphosphonate compounds, and physical activity programs [10]. Several researchers have determined that continuous wave (CW) low-level laser therapy (LLLT) stimulates in vitro mineralization through increased IGF-I and BMP production, Runx2 expression, and ERK phosphorylation [11]. CW LLLT has been shown to stimulate bone nodule formation [12] in osteoblasts. Others reported that LLLT promoted the acceleration of bone strength and consolidation after a fracture, created new blood vessels, increased collagen fiber deposition, and promoted greater bone cell proliferation at the fracture site [13, 14]. Pinheiro et al. reported that LLLT resulted in increased mineralized bone tissue in the fractured femora [15]. Bossini et al. concluded that LLLT improved bone repair in the tibia of osteoporotic rats by stimulation of newly formed bone, fibrovascularization, and angiogenesis [16].
Studies of the influence of CW low-level lasers on ovariectomized-induced OP (OVX-d) in cortical bone have been few and with inconsistent results [17–21]. Ko et al. [17], Ko et al. [18, 19], and Renno et al. [20] reported that LLLT of osteoporotic bones produced beneficial effects on the prevention of bone loss. However, Medalha et al. [21], in a recent study, indicated that treatment of femurs and tibias of spinal cord injured rats with an 830-nm laser showed a trend toward significantly higher values of internal and external areas of tibial diaphysis. No increase was found in either mechanical or densitometric parameters. Consistently, Renno et al. reported that LLLT did not improve the stimulatory effects of the exercise on the osteopenic rats. [22].
The application of frequency is growing. A literature review produced three cellular studies on rat calvarial cells [23–25], one in vivo study on the tooth movement speed of rat molars [26], a study on bone turnover in ovariectomized rats [27], and a study on healing of a partial osteotomy of the tibia in streptozotocin-induced diabetic rats [28].
The aim of this study was to assess the effects of PW LLLT on cortical bone (tibia) strength of the following: (1) normal, healthy rats; (2) an OVX-d experimental model in rats; and (3) a GIOP experimental model in rats. Bone strength was evaluated by measuring the biomechanical properties of tibial diaphysis, which included bending stiffness (Young’s modulus of elasticity), maximum force, and stress high load using a three-point bending test instrument.
Materials and methods
Experimental animals
A total of 66 adult male and female Wistar rats, aged 4.5 months were housed in standard rat cages on a 12-h light/dark schedule and provided water ad libtum. All procedures were approved by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (protocol no 1392-1-115-1160). The ratsʼ body weights were monitored weekly, and the volume of drugs administrated was calculated according to the most recent body weight.
Experimental protocols
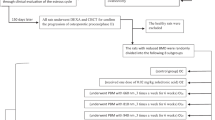
In this study, normal, OVX-d, and GIOP rats received LLLT and alendronate, after which they were subjected to a three-point bending test.
Normal rats
There were 12 male rats divided equally into control and experimental groups. The right tibias of the experimental (laser-treated) rats were exposed to a pulsed infrared diode laser [MUSTANG 2000+ with LO7 pen (radiating head), Technica Co., Moscow, Russia] with the specifications shown in Table 1. During laser irradiation, irradiated animals were sedated by ketamine (25 mg/kg) and diazepam (2.5 mg/kg) anesthesia. Control rats received a placebo PW LLLT that was switched off. In the current study, the surface area of the bone was larger than the pen’s spot size; therefore, we used sequential treatments (four shootings of the laser) to ensure that all areas of the bone received similar laser doses [29].
Ovariectomized-induced osteoporosis (OVX-d) and glucocorticoid-induced osteoporosis (GIOP) groups
The remaining 54 rats were randomly divided into 9 groups of 6 rats each. The groups consisted of the following: four OVX-d groups, four groups that received dexamethasone, and one healthy group considered for baseline studies (group 7). Ovariectomy was performed by two paravertebral skin incisions while rats were anesthetized with ketamine (50 mg/kg) and diazepam (5 mg/kg) anesthesia. The uterine tubes were ligated (catgut 4.0), and following removal of the ovaries, we closed the incisions (nylon 3.0). Ceftriaxone (Jaber ben Hayan, Tehran, Iran) at a dose of 50 mg/kg was administrated immediately before and 24 and 48 h after surgery. All animals were kept for 14 weeks after surgery in cages in order to develop OP [30]. At the end of this period, the rats were submitted to different treatments as follows: group 3 (OC) comprised control rats with OP; group 4 (OA) were OVX-d rats treated subcutaneously with 1 mg/kg alendronate (Alborz Darou, Tehran, Iran); group 5 (OL) consisted of OVX-d rats treated with LLLT three times weekly (Table 1); and group 6 (OAL) were OVX-d rats treated with LLLT and concomitant administration of alendronate.
The remaining rats received intramuscular injections of dexamethasone (Alborz Darou, Tehran, Iran) at a daily dose of 1 mg/kg, 6 days per week for 5 weeks [31], with some modifications, i.e., 6 days per week instead of 7 days per week, and 5 weeks instead of 4 weeks. After 5 weeks, dexamethasone-treated rats were divided into four groups: group 8 (control) was OP rats treated with intramuscular injections of vehicle (distilled water; DC); group 9 (DA) or GIOP rats were treated with subcutaneous injections of 1 mg/kg alendronate (Alborz Darou, Tehran, Iran) [32]; group 10 (DL) was GIOP rats treated with PW LLLT (Table 1) three times per week; and group 11 (DAL) consisted of GIOP rats treated with LLLT and concomitant administration of 1 mg/kg/day alendronate (Table 2). In the laser-treated rats, both tibias (four points each) were irradiated with the laser pen held perpendicular to the bone from a distance below 1 cm. LLLT was administered for a period of 8 weeks, and alendronate injections were given for 30 days.
General examinations
At 8 weeks after the beginning of the treatments, we killed the rats with an overdose of anesthesia. Blood glucose concentrations were measured using distal tail vein blood samples (GM 300, Biomince, GMH, Heerbrugg, Switzerland). The right tibias were extracted and left tibias frozen for further examinations. The length of the tibia (mm) was measured using a sliding caliper, and the weight of the tibia was measured in grams.
Biomechanical examinations
The bones were subjected to three-point bending on a material testing device (Zwick/Roell, Germany) until fracture, defined as separation of the bone into two pieces occurred. All the bones were oriented similarly in the testing machine, and we calculated each bone’s surface area. Two loading points, 19 mm apart, were used to mount each bone, and a press head was subsequently activated to compress the midline of the bone shaft until a fracture occurred. The compressive loading speed was 0.08 mm/s for all tests. Specimens were loaded uniaxially so that the fracture and complete load-deformation curve could be recorded by transducers coupled to bridges and sampled in a personal computer by an analog-to-digital convertor (PC-software 27005). Load characteristics were directly plotted on an x-y chart recorder. From the load-deformation curve, the following biomechanical properties were automatically calculated: bending stiffness (N/mm), maximum force (N), and stress high load (N/mm2). We defined these biomechanical parameters as follows. Bending stiffness is the slope of the linear portion of load-deformation curve (i.e., the ratio of loading to deformation in the elastic region of the curve). Maximum force is the force needed to break the bone microscopically. The stress high load was calculated by dividing the maximum force value by surface area (mm2) of the bone at the site of the fracture [33].
Statistical analysis
All data were expressed as mean ± standard errors of mean (SEM).
Normal distribution of data was analyzed using the one-sample Kolmogorov-Smirnov test. Parametric and nonparametric statistical methods were used. Significance of differences between the normal control and laser-treated normal groups was assessed using the Student’s t test. The analysis of variance (ANOVA) test was used to compare changes among groups with normal distribution of data and LSD’s test to identify differences. A p value of ≤0.05 was considered statically significant. Nonparametric methods were used for statistical analysis of other groups. These data were analyzed using the Kruskal-Wallis and Mann-Whitney U tests. Differences were regarded as significant if p < 0.005 for analyses between groups 3–11. The differences were also regarded as significant if p ≤ 0.01 for analyses between groups 3–7 and analyses between groups 7–11, respectively.
Results
Normal rats
The independent sample t test showed a significantly lower maximum force in the control group (25 ± 1.3) compared to the experimental group (37.3 ± 3.6; p = 0.01).
Ovariectomized-induced osteoporosis (OVX-d) and glucocorticoid-induced osteoporosis (GIOP) rats
General observations
Blood glucose
All blood glucose values were less than 120 mg/dc. According to the ANOVA test, there were no significant differences in blood glucose values between the studied groups (Table 3).
Body weight
As seen in Table 4, the use of dexamethasone led to a significant decrease in body weight (p < 0.001). Although rats from all groups that received dexamethasone lost body weight, the weight loss was more severe in rats from groups DC and DL. In all groups, five rats died during dexamethasone administration, and three rats died during alendronate treatment and LLLT. In the OVX-d groups, four rats died during the study. The dead rats were replaced. The OA group showed a significant increase in body weight (p < 0.01).
Tibia length
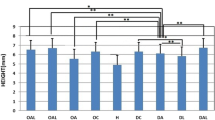
According to the LSD test, the tibial lengths of the OA (p = 0.011), OL (p = 0.009), OAL (p = 0.012), DC (p = 0.023), DA (p = 0.002), DL (p = 0.001), and DAL (p = 0.015) groups significantly increased compared to the healthy group. DC group showed a significant increase in tibial length compared to the DA (LSD test, p = 0.041) and OL (p = 0.004) groups. There were significantly greater tibial length values in the DL group compared to the DA (p = 0.23), OC (p = 0.039), OAL (p = 0.000), and OL (p = 0.003) groups (LSD test; Fig. 1). Laser treatment administered to dexamethasone-treated rats had a positive effect on tibial length.
Tibial weight
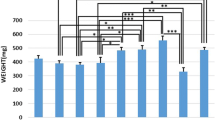
Tibial weights of all groups, with the exception of the OC group, significantly increased compared to the healthy group. The OC group had a significant decrease in tibial weight compared to the OL (LSD test, p = 0.022), OA (p = 0.025), OL (p = 0.028), DL (p = 0.001), DA (p = 0.004), DAL (p = 0.033), and DC (p = 0.05) groups. The DA group showed a significant increase in tibial weight compared to the DC (p = 0.049) and OC (p = 0.004) groups. All results are shown in Fig. 2.
Mean ± SEM of tibial weight (gram) of studied groups compared by LSD test; * <0.05, ** <0.01. According to the LSD test, the OC group had a significant decrease in tibial weight compared to the OA, OL, DL, DA, DAL, and DC groups. The DA group showed a significant increase in tibial weight compared to the DC and OC groups
Biomechanical results
The results of biomechanical analyses are shown in Figs. 3, 4, and 5.
Mean ± SEM of tibial stress high load (N/mm2) of studied groups compared by Mann-Whitney test; ** <0.01. Stress high load values of the OA and DA groups were significantly higher than the DL group (Mann-Whitney test). LLLT did not have any positive effect on maximum force or stress high load of osteoporotic bones
Bending stiffness
There was a significant increase in bending stiffness in all study groups compared to the healthy group (Mann-Whitney test, p = 0.004 for all groups). The DA group showed significant increase in bending stiffness compared to the DA and DAL groups (both p = 0.004). LLLT had no positive effect on bending stiffness of osteoporotic bones. All results are shown in Fig. 3.
Maximum force
According to the LSD test, the maximum force observed in all groups significantly increased (OC, OL, DC, and DL: p = 0.000 for all; OA and OAL: p = 0.002 for both; DL: p = 0.006) compared to the healthy group. The DA group showed a significant increase in maximum force compared to the DL group (p = 0.035). LLLT did not show any positive effect on the maximum force of osteoporotic bones. All results are shown in Fig. 4.
Stress high load
There was a significant increase in the OAL group for stress high load compared to the healthy, DL, and DAL groups (Mann-Whitney test, p = 0.004 for all). Stress high load values for the OA and DA groups were significantly higher than the DL group (Mann-Whitney test, p = 0.004). LLLT had no positive effect on the stress high load. As seen in Fig. 5, LLLT did not show any positive effect on the stress high load of osteoporotic bones.
Discussion
In the current study, we observed significant body weight loss in GC administered groups which resulted in a considerable mortality rate of 10 % among the rats. We observed significantly higher weights and biomechanical properties of the tibia from control rats treated with dexamethasone compared to healthy rats. With respect to the increased bone weights and biomechanical properties of dexamethasone-treated rats, we concluded that these findings might be attributed to the anabolic effects of GC administration at the level of the cortical bone [34, 35].
These data contradicted findings in patients with supraphysiologic GC administration. Those patients had OP, bone loss, increased bone fractures, and increased body weight [1, 36].
OP is a cause of bone loss and fractures which can lead to severe pain, deformity, and in certain cases, secondary complications that result in death [1]. Therefore, it is important to investigate treatments that have osteogenic potential, the capability to stimulate bone formation, and prevent bone loss [22]. The osteogenic effects of PW LLLT on osteoblastic cell proliferation and bone metabolism are well known [23–27].
Previous studies have shown that in vitro application of PW LLLT on bone cells stimulates bone formation [23, 25], promotes osteoblast proliferation and differentiation, and indirectly inhibits osteoclast differentiation by downregulation of the RANKL/OPG mRNA ratio in osteoblasts [24]. Thus, PW LLLT irradiation may play an important role in bone remodeling and should be a valuable option for the treatment of bone diseases such as OP. In vivo studies have shown that PW LLLT treatments led to faster orthodontic tooth movement [26], effectively enhanced bone formation, and decreased bone resorption in osteoporotic OVX-d rats [27].
In contrast, PW LLLT at an 80-Hz pulse frequency, 900-s duration, 0.972-J/cm2 energy density, and other delivery parameters used in the current study failed to cause any beneficial effects in the biomechanical parameters of osteoporotic bones. These results were supported by other studies [21, 22].
Medalha et al. found no increase in either mechanical or densitometric parameters of laser (830 nm, 250 J/cm2)-treated spinal cord-injured rats’ bones [21]. Renno et al. showed that CW LLLT at 120 J/cm2 did not enhance the effect of exercise on the femora of OVX-d rats. They concluded that since LLLT has osteogenic properties, higher delivery parameters should be investigated to verify if increased energy could create an extra-osteogenic stimulus in addition to exercise [22].
Possibly, the stimulatory effects of the PW LLLT protocol used in the current study was insufficient in decreasing the detrimental effects of the experimental OP induced by dexametasone and the ovarieoctomy. Furthermore, the combination of alendronate (1 mg/day) and PW LLLT in OVX-d rats partially preserved bone function and formation. This combination significantly increased the stress high load of the osteoporotic tibia compared to the healthy group. The results of this study confirmed data from a previous study [37]. Diniz et al. investigated the effect of CW LLLT in combination with bisphosphonate on osteoporotic trabecular bone structure [37]. Laser-treated rats received a gallium-aluminum-arsenium laser at 830 nm, 50 m Watt, and 4 J/cm2 on the femoral neck and vertebral segments. Remarkably, in the association between laser and alendronate, the trabecular bone volume was significantly greater in the vertebrae. They concluded that LLLT associated with alendronate administration was the best method for reversing vertebral OP caused by the ovariectomy [37].
We recently studied the capability of PW LLLT to increase bending stiffness in repairing an osteochondral defect [38]. The experimental group received PW LLLT at a frequency of 1500 Hz and energy density of 4.8 J/cm2. The defects were examined biomechanically. There was significantly higher equilibrium indentation stiffness observed in the experimental group compared to the control group. Kamali et al. have concluded that PW LLLT significantly enhanced the stiffness of repairing tissue at the 8-week postinjury in rabbits with osteochondral defects [38]. Bending stiffness reflects the mineral fraction of the bone [39]. It seems that the effect of LLLT on tissue is dependent both on energy density and other parameters [40]. The PW LLLT devices provide more laser (light) parameters than CW LLLT devices. It is assumed that by investigating different values of these parameters, research models can be more effectively studied and compared with CW LLLT devices in an attempt to achieve better outcomes [41–43].
Although we observed better biomechanical results in the DA group compared to other dexamethasone-treated rats, these differences were not significant. Possibly, if the amount of alandronate administration to the rats was adequate, there would be significant changes between those groups.
Conclusion
PW LLLT at the parameters used in this study failed to cause any beneficial biomechanical effects in the examined osteoporotic cortical bones. PW LLLT associated with alendronate treatment produced a more remarkable effect on bone strength in the ovariectomized induced OP model in rats.
Further studies that investigate different values of PW LLLT delivery parameters are needed to demonstrate the probable positive effects of PW LLLT on preventing bone loss in osteoporotic bones.
References
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Pitterson C, de Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
U.S. Department of Health and Human Services (2004) Bone health and osteoporosis: a report of the Surgeon General. U.S. Department of Health and Human Services, Office of the Surgeon General, Rockville
(2009) Chapter 1: a public health approach to promote bone health. Available at: http://www.surgeongeneral.gov/library/bonehealth/chapter_1.html#TheMagnitudeoftheProblem. Accessed 14 Sept 2009
Riggs BL, Khosla S, Melton LJ 3rd (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302
Eriksen EF, Hodgson SF, Eastell R, Cedel SL, O’Fallon WM, Riggs BL (1990) Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res 5:311–319
Kim H-J (2010) New understanding of glucocorticoid action on bone cells. BMB Rep 43:524–529
Egerman M, Goldhahn J, Schneider E (2005) Animal models for fracture treatment in osteoporosis. Osteoporos Int 16:S 129–S 138
Peng Z, Tuukkanen I, Zhang H, Jamsa T, Vaananen HK (1994) The mechanical strength of bone in different rat models of experimental osteoporosis. Bone 15:523–532
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Office of the Surgeon General (US) (2004) Bone health and osteoporosis: a report of the Surgeon General. Office of the Surgeon General (US), Rockville
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, de Araújo HS, Renno AC (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed Laser Surg 29:311–317
Kiyosaki T, Mitsui N, Suzuki N, Shimizu N (2010) Low-level laser therapy stimulates mineralization via increased Runx2 expression and ERK phosphorylation in osteoblasts. Photomed Laser Surg 28(Suppl 1):S167–S172
Shimizu N, Mayahara K, Kiyosaki T, Yamaguchi A, Ozawa Y, Abiko Y (2007) Low-intensity laser irradiation stimulates bone nodule formation via insulin-like growth factor-I expression in rat calvarial cells. Lasers Surg Med 39:551–559
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22:97–102
Trelles MA, Mayaio E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Pinheiro AL, Limeira Júnior Fde A, Gerbi ME, Ramalho LM, Marzola C, Ponzi EA, Soares AO, De Carvalho LC, Lima HC, Gonçalves TO (2003) Effect of 830-nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseus membrane. J Clin Laser Med Surg 21:301–306
Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A, Parizotto NA (2012) Low level laser therapy (830nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol 47:136–142
Ko CY, Kang H, Ryu Y, Jung B, Kim H, Jeong D, Shin HI, Lim D, Kim HS (2013) The effects of minimally invasive laser needle system on suppression of trabecular bone loss induced by skeletal unloading. Lasers Med Sci 28:1495–1502
Ko CY, Kang H, Seo DH, Jung B, Schreiber J, Kim HS (2013) Low-level laser therapy using the minimally invasive laser needle system on osteoporotic bone in ovariectomized mice. Med Eng Phys 35:1015–1019
Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) Effects of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Surg 24:642–645
Medalha CC, Amorim BO, Ferreira JM, Oliveira P, Pereira RM, Tim C, Lirani-Galvão AP, da Silva OL, Renno AC (2010) Comparison of the effects of electrical field stimulation and low-level laser therapy on bone loss in spinal cord-injured rats. Photomed Laser Surg 28:669–674
Muniz Renno AC, de Moura FM, dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) The effects of infrared-830 nm laser on exercised osteopenic rats. Lasers Med Sci 21:202–207
Ueda Y, Shimizu N (2001) Pulse irradiation of low-power laser stimulates bone nodule formation. J Oral Sci 43:55–60
Xu M, Deng T, Mo F, Deng B, Lam W, Deng P, Zhang X, Liu S (2009) Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed Laser Surg 27:309–315
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21(5):271–277
Duan J, Na Y, Liu Y, Zhang Y (2012) Effects of the pulse frequency of low-level laser therapy on the tooth movement speed of rat molars. Photomed Laser Surg 30:663–667
Saad A, El Yamany M, Abbas O, Yehia M (2010) Possible role of low level laser therapy on bone turnover in ovariectomized rats. Endocr Regul 44:155–163
Javadieh F, Bayat M, Abdi S, Mohsenifar Z, Razi S (2009) The effects of infrared low-level laser therapy on healing of partial osteotomy of tibia in streptozotocin-induced diabetic rats. Photomed Laser Surg 27:641–646
Bayat M, Abdi S, Javadieh F, Mohsenifar Z, Rashid MR (2009) The effects of low-level laser therapy on bone in diabetic and non diabetic rats. Photomed Laser Surg 27:703–708
Li X, Ominsky MS, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Dwyer D, Grisanti M, Stolina M, Kostenuik PJ, Simonet WS, Paszty C, Ke HZ (2011) Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology 152:3312–3322
Ferretti JL, Gaffuri O, Capozza R, Cointry G, Bozzini C, Olivera M, Zanchetta JR, Bozzini CE (1995) Dexamethasone effects on mechanical, geometric and densitometric properties of rat femur diaphyses as described by peripheral quantitative computerized tomography and bending tests. Bone 16:119–124
Sun P, Cai DH, Li QN, Chen H, Deng WM, He L, Yang L (2010) Effects of alendronate and strontium ranelate on cancellous and cortical bone mass in glucocorticoid-treated adult rats. Calcif Tissue Int 86:495–501
Abdi S, Bayat M, Javadieh F, Mohsenifar Z, Rezaie F, Bayat M (2009) The effects of helium-neon light therapy on healing of partial osteotomy of the tibia in streptozotocin induced diabetic rats. Photomed Laser Surg 27:907–912
King CS, Weir EC, Gundberg CW, Fox J, Insogna KL (1996) Effects of continuous glucocorticoid infusion on bone metabolism in the rat. Calcif Tissue Int 59:184–191
Henneicke H, Hermann M, Kalak R et al (2011) Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone 49:733–742
Mc Donough AK, Curtis JR, Saag KG (2008) The epidemiology of glucocorticoid-assotiated adverse events. Curr Opin Rheumatol 20:131–137
Diniz JS, Nicolau RA, de Melo Ocarino N, do Carmo Magalhaães F, de Oliveira Pereira RD, Serakides R (2009) Effect of low-power gallium-aluminum-arsenium laser therapy (830 nm) in combination with bisphosphonate treatment on osteopenic bone structure: an experimental animal study. Lasers Med Sci 24:347–352
Kamali F, Bayat M, Torkaman G, Ebrahimi E, Salavati M (2007) The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. The therapeutic effect of low-level laser on repair of osteochondral defects in rabbit knee. J Photochem Photobiol B 27(88):11–15
Einhorn TA (1992) Bone strength: the bottom line. Calcif Tissue Int 51:333–339
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Bayat M (2014) The necessity for increased attention to pulsed low-level laser therapy. Photomed Laser Surg 32:427–428
Ezzati A, Bayat M, Khoshvaghti A (2009) Low-level laser therapy with a pulsed infrared laser accelerates second-degree burn healing in rat: a clinical and microbiologic study. Photomed Laser Surg 28:603–611
Vasheghani MM, Bayat M, Dadpay M, Habibie M, Rezaei F (2009) Low-level laser therapy using 80-Hz pulsed infrared diode laser accelerates third-degree burn healing in rat. Photomed Laser Surg 27:959–964
Acknowledgments
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei. We wish to express our appreciation to the Vice Chancellor of Research at Shahid Behesti University of Medical Sciences for financial support (grant no. 1392-1-115-1160).
Conflict of interest
No competing financial interests exist.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fridoni, M., Masteri Farahani, R., Nejati, H. et al. Evaluation of the effects of LLLT on biomechanical properties of tibial diaphysis in two rat models of experimental osteoporosis by a three point bending test. Lasers Med Sci 30, 1117–1125 (2015). https://doi.org/10.1007/s10103-014-1706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1706-1