Abstract
This work evaluated the anti-inflammatory response of low-power light-emitting diode (LED) and ultrasound (US) therapies and the quality and rapidness of tendon repair in an experimental model of tendinitis, employing histomorphometry and Raman spectroscopy. Tendinitis was induced by collagenase into the right tendon of 35 male Wistar rats with an average weight of 230 g. The animals were randomly separated into seven groups of five animals each: tendinitis without treatment—control (TD7 and TD14, where 1 and 2 indicated sacrifice on the 7th and 14th day, respectively), tendinitis submitted to US therapy (US7 and US14) and tendinitis submitted to LED therapy (LED7 and LED14). Contralateral tendons of the TD group at the 14th day were used as the healthy group (H). US treatment was applied in pulsed mode at 10 %, 1 MHz frequency, 0.5 W/cm2, 120 s. LED therapy parameters were 4 J/cm2, 120 s, daily dose at the same time and same point. Sacrifice was performed on the 7th or 14th day. Histomorphometric analysis showed lower number of fibroblasts on the 14th day of therapy for the US-treated group, compared to the TD and LED, indicating lower tissue inflammation. Raman showed that the LED group had an increase in the amount of collagen I and III from the 7th to the 14th day, which would indicate more organized fibers and a better quality of the healing, and US showed lower collagen I synthesis in the 14th day compared to H, indicating a lower tissue reorganization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tendinitis is an inflammatory condition of the tendon and this acute inflammatory condition triggers the process of tissue repair [1]. However, this process is often exaggerated and can lead to complications that impair the quality of the healed tissue [2]. Tendon injuries are common in Sports and are estimated to correspond to 30 % to 50 % of these injuries [2–4]. According to Aglietti and co-workers [5], tendinitis is one of the main problems related to repetitive force injury and work-related musculoskeletal disorders. Several studies indicated that tendinitis is the major cause of suffering of manual labourers, as well as workers' compensation [6].
Several techniques have been used in physiotherapy in order to promote tissue repair. Therapeutic high-frequency ultrasound (US) is the technique of choice for tendinitis treatment. The US apparatus generates high-frequency electric current and is connected to a piezoelectric synthetic ceramic lead zirconate titanate, housed in a transducer [7–10]. The US technique has been used clinically to promote tissue regeneration and repair, increase protein synthesis and blood flow, normalise muscle tone, activate calcium cycle, stimulate nerve fibres and peripheral nerves, increase mobility of articulation, increase extensibility in collagen-rich tissues, reduce muscle spasms, relief pain, and resolve chronic inflammatory processes [11–14]. On the other hand, light emitting diode (LED) has been proposed for tissue repair [15]. LED is a continuous, incoherent light source with high luminescent efficiency and low cost, produced by a semiconductor diode that, when powered, emits infrared or visible light [16]. Studies have been demonstrated the use of light in healing cicatrisation tendinitis [17, 18].
The healing effect stimulated by light is characterised by three main factors. The first factor is the increase in the production of ATP [19, 20]. The second is through the stimulation of microcirculation; the third is the formation of new vessels from existing ones [20, 21].
Evaluation of bone and tendon tissue alterations and repair, induced by therapeutic techniques such as US and LED, is traditionally done by ultrasound and X-ray examinations. Recently, several authors have proposed the use of optical techniques such as Raman spectroscopy for a minimally invasive and non-destructive diagnosis of tendons and bones [21–24]. Raman spectroscopy is a fundamental process of energy exchange between incident light and matter, which depends on changes in the molecular polarizability when irradiated by a light wave, resulting in inelastic scatter of the incident light by the molecules [25, 26]. Raman technique can be used to characterise biochemical changes in various disorders, correlating the spectroscopic findings with the clinical diagnosis and quantifying the relative amount of selected biochemicals, such as proteins, lipids and nucleic acids in the biotissues [27, 28], and enabling an effective therapeutic evaluation in real time [26].
Raman spectroscopy can provide an estimation of the changes related to organic and inorganic compounds in the region of the tendon–bone insertion, interpretation of the relationship between mineral/collagen at individual points along the length of the bone–tendon, using the spectral information provided by the Raman peaks proteins and inorganic (mineral) matrix [20], and in the biochemical changes in normal and degenerate tendon tissues. These measures are determined by measuring the spectral differences related to the different concentrations of amino acids from proteins (glycine, proline, hydroxyproline, phenylalanine, tyrosine mainly from collagen I and III) and lipids, which are well correlated with hyaline state and cellular activity attributed to biochemical abnormalities due to the degenerative process suffered by the tendon [23].
The estimation of the biochemistry in biological samples by means of Raman spectroscopy can be assessed using least-square-based linear models that use the spectral information from basal constituents such as proteins (e.g. collagens, elastin or actin), lipids (e.g. triolein) and phospholipids, among others fitted to the spectra of tissues, assuming that each basal compound has a linear contribution to the observed spectrum [29]. By applying basic statistics, one can correlate the amount of collagen measured by classical histochemistry imaging technique, with the quantities estimated by the model using the Raman spectra.
This study intended to evaluate the anti-inflammatory and tissue repair effects in an experimental model of tendinitis using therapeutic high-frequency ultrasound (US) [2], and low-power light emitting diode (LED) [30, 31], by means of histopathologic and histomorphometric analyses, trying to corroborate the histological and morphometrical results with the information provided by the FT-Raman spectra of tendons, by spectrally evaluating the relative amount of collagen deposited in the tendon estimated by a spectral model that uses the spectra of collagen I and III linearly fitted to the spectra of tendons.
Material and methods
Animals and experimental groups
All experiments were carried out in accordance with the guidelines for animal care following the guidelines of COBEA (Brazilian College of Animal Experimentation) and were approved by the ethics committee of the Faculdade Integral Diferencial-Teresina, Piauí, Brazil (protocol no. 086/09). The experimental model involved 35 male Wistar rats (Rattus norvegicus, albinus), 30 days old, average weight 230 g and were maintained under controlled room temperature 24 ± 1 °C and humidity of 60 %, photoperiod 12-h light/dark, and fed standard palletized food and water ad libitum. These animals were maintained during the experimental period collectively in properly labelled, standard polyethylene cages with five animals per cage.
The animals were first anaesthetised with ketamine (100 mg/kg, intraperitoneal) and xylazine (20 mg/kg, intraperitoneal). Then tendinitis was experimentally induced by intratendinous injection of 10 μL of collagenase (10 mg/mL, Sigma, C6885, diluted in sterile-buffered saline phosphate) into the right tendon of the animals. The animals received the injection of collagenase on day 1, and then were into seven experimental groups with five animals each (35 animals), as described below (Table 1):
-
TD: after the experimental induction of tendinitis, the animals were kept in cages without any treatment and sacrificed at the 7th (TD7) or 14th (TD14) day
-
US: after the experimental induction of tendinitis, the rats were treated daily with ultrasound in pulsed mode at 10 %, with a frequency of 1 MHz, 0.5 W/cm2 for 120 s and sacrificed at the 7th (US7) or 14th (US14) day
-
LED: after the experimental induction of tendinitis, the rats were given daily LED therapy with an output of 4 J/cm2 and wavelength of 620 ± 20 nm for 120 s and sacrificed at the 7th (LED7) or 14th (LED14) day
-
H: healthy group; this group was composed of the contralateral leg tendons of the animals from the TD group sacrificed on the 14th day
At the day of sacrifice, animals received an overdose of anaesthetic (thiopental sodium) and tendons were dissected, withdrawn, snap-frozen and stored in liquid nitrogen for spectral and histological analysis.
US and LED therapy
The application of therapy in the experimental groups (US or LED) started 24 h after induction of tendinitis and was repeated by the same person daily at the same hour until euthanasia. For the therapeutic procedure, the animals were in the prone position lying on a bench covered with a non-textured fabric. The right foot was manually immobilised while the prescribed therapy was applied directly to the incision. The US device was a Sonopulse Special (827 series, IBRAMED, São Paulo, Brazil) with a water-based coupling gel (RMC, IBRAMED). The execution was directly coupled with the oscillatory movements in an effective radiation area of 1 cm2, and the device was calibrated by the manufacturer. The LED used was assembled in our laboratory, which possesses a tip with the peak wavelength band of 640 nm, average power of 30 mW and spot area of 0.5 cm2, with manual control of the shutter. The procedure was done following the same protocol as the US, with the device positioned 90° from the tendon in order to optimise the irradiation (absorption and scattering) without significant loss of energy and providing better contact with the animal's leg.
Histology and histomorphometry
All animals from each group were euthanized on the 7th or 14th day after the experimental induction of tendinitis. The tendons were then removed by tissue dissection of the right leg, starting the first incision in the tendon bone region and the second incision in the muscle–tendon insertion. The tendons of the left paw of the TD14 group (contralateral, without induction or treatment) was dissected and considered the healthy group.
The histological analysis was done by a board-certified pathologist. The tendons were longitudinally bisected and then treated according to conventional histology procedure in an automatic tissue processor. Semi-serial, 5-μm thick cuts were obtained from a microtome, and two were stained with haematoxylin and eosin and two with Masson’s trichrome (MT), which allows the visualisation of collagen fibres. The slides were examined with an optical microscope (model CX31, Olympus, Japan, with 100X objectives), equipped with a digital camera (model SC20, Olympus) and connected to a microcomputer.
The histomorphometry was performed on slides stained with MT to quantify the number of fibroblasts. From each slide two microscopic fields were captured (400X) where a higher cell concentration was seen. The scanned images were then evaluated with the aid of the analysis program ImageJ ® (version 1.32 for Windows, public domain, National Institute of Health, Bethesda, MD, USA). The data were presented as mean and standard deviation (mean ± SD).
The data were tested for normality using the Kolmogorov–Smirnov test to determine the statistical test to be used, considering the level of statistical significance of 5 % (p < 0.05). The statistical analysis of the differences in the number of inflammatory cells found in the experimental groups was calculated using the ANOVA test followed by the Tukey–Kramer, using the statistical software GraphPad Instat® version 3.0.
Raman spectroscopy
Spectra were collected using an FT-Raman spectrometer (RFS 100/S, Bruker Optik GmbH, Ettlingen, Germany) at the Laboratory of Biomedical Vibrational Spectroscopy–UNIVAP. The samples were passively warmed to reach room temperature and kept in 0.9 % saline solution until ready to be placed in the spectrometer. The time taken handling the unfrozen sample was not longer than 15 min, to avoid dehydration. The samples were then placed in an aluminium sample holder.
The FT-Raman spectrometer uses a Nd:YAG laser with a 1,064-nm wavelength and approximately 200-mW laser power in the sample, in order to avoid heating or sample degradation. The acquisition parameters were set to achieve a resolution of 4 cm−1 (200 scans, 5-kHz sweep and 7-mm slit aperture) in the spectral range of 350 to 1,800 cm−1. Triplicate spectra were obtained in each sample, with a total of 105 Raman spectra for all 35 samples.
In order to perform a quantitative analysis of changes in the amount of proteins in the tendons depending on the treatment (US/LED) compared to controls, spectra were submitted to a spectral model that calculates the relative amount of basal constituent collagen I, collagen III and triolein in each Raman spectrum of tissue using the spectra of pure compounds (collagen I, C7774; collagen III, C4407; and triolein, T7140; Sigma-Aldrich, Inc.). These constituents are the major contributors to the observed spectra of tendons and are indicative of tissue remodelling. Triolein was included in the model due to its spectral contribution in some samples.
This model uses an ordinary least-square fitting of the spectra of constituents to the spectra of the tendons, where the fitting coefficients are related to the Raman concentration of the specific basal constituent in the particular spectrum [27]. The model uses the expression:
where A is the tissue spectrum to be estimated, SC is the matrix of spectral constituents, C is the matrix of Raman concentrations to be predicted by the least-square fitting and E is the error fitting. This can be used to provide a fitting of the spectral constituents believed to belong to the tissue found within the measured spectrum, assuming that the residual is minimised and that the spectral components selected are the main components of the spectra [27].
Results
Histological assessment
In the H group (Fig. 1), the tendons showed a normal histological aspect: elongated fibrocytes (tenocytes) with scarce cytoplasm and fusiform nucleus, sparsely found among dense parallel bundles of collagen. The epitendon and endotendon were visible as thin bands of looser connective tissue containing small blood vessels.
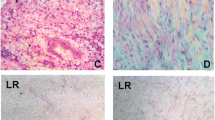
Photomicrographs indicating histological aspects of the tendon of the rats in group H (healthy). a Collagen fibres are disposed in longitudinal, compact bundles, with scarce permeating tenocites. b, c The epitendon contains little and delicated blood vessels, elongated cells randomly disposed and low density matrix. d The endotendon presented grooves similar to the epitendon, but thinner and situated at the middle of the tendon. a, b, d H & E, X400; c Masson’s trichrome, X400
In the TD7 group (Fig. 2a–d), it was found that there was a thickening of the epitendon and endotendon due to the presence of granulation tissue, formed by a loose extracellular matrix containing small newly formed vessels, mononucleated inflammatory cells and numerous fibroblasts. Neutrophils were not identified. Fibroblasts were voluminous with an abundant cytoplasm and large nucleus, found in a random pattern, without forming well-defined bundles or being longitudinally oriented. The extracellular matrix had a fibrillar aspect, compared with the residual fibres of the tendon, which was looser with none or only a weak positive reaction for Masson's trichrome. In the TD14 group (Fig. 2e–h), it was found that mononuclear inflammatory cells and newly formed vessels in the epitendon and endotendon apparently decreased, while the amount of fibroblasts remained high. The closer to the existing tendon was the proliferation of fibroblasts, the greater the tendency of these layers to be aligned in parallel. In these areas, the extracellular matrix was dense, but without the same intensity of Masson's staining observed in the undamaged areas. In no animals were the damaged sections remodelled into normal tendon structure.
Photomicrographs indicating histological aspects of the tendon of the animals in groups TD7 (tendinitis, 7th day, (a–d)) and TD14 (tendinitis, 14th day (e–h)). a, b The epitendon is enlarged due to the vascular neoformation, presence of fibroblasts and mononuclear inflammatory cells. c, d Presence of newly formed blood vessels and voluminous fibroblasts randomly disposed. Similar changes were also found in the endotendon. e, f the epitendon is still enlarged with numerous fibroblasts; it can be seen a tendency of parallel alignment of these cells with the non-affected tendon seen in the inferior part of the picture. g, h the extracellular matrix exhibits more dense portions, but without the same color pattern as the healthy tendon. a, e H & E, X100; b, f Masson’s trichrome, X100; c, g H & E, X400; d, h Masson’s trichrome, X400
In the US7 group (Fig. 3a–d), it was found that both the epitendon and endotendon expanded by increasing the number of tenoblasts and extracellular matrix, which although presenting a fibrillar aspect did not show the same intensity of staining by Masson's trichrome as observed in uncompromised areas. Compared to the TD7 group (Fig. 2a–d), the mononuclear inflammatory cells appeared in smaller numbers, and the newly formed vessels showed thicker walls. The tenoblasts were apparently more numerous, generally had greater progression of organisation and formed more defined bundles, especially in areas close to the existing collagen bundles. In the US14 group (Fig. 3e–h), it was found that the number of newly formed vessels diminished greatly and inflammatory mononuclear cells were no longer found. The tenoblasts remained numerous, but thinner and with less cytoplasm. The extracellular matrix was dense and stained with Masson's trichrome and had characteristics much like those of the pre-existing fibres.
Photomicrographs indicating histological aspects of the tendon of the animals in the groups US7 (US, 7th day (a–d) and US14 (US, 14th day (e–h)). a, b It can be seen numerous fibroblasts arranged in well-defined bundles in the epitendon; inflammatory cells are scarce and the neoformed vessels have thicker walls. c, d It can be seen as a tendency of the neoformed tenocytes to align with the existing ones. e, f Newly formed fibroblasts are arranged in parallel bundles in the epitendon. g, h Thin fibroblasts separated by dense extracellular matrix and with a staining pattern similar to that observed in the original tendon; inflammatory cells are not seen and blood vessels are scarce. a, e H & E, X100; b, f Masson’s trichrome, X100) (c, g H & E, X400; d, h Masson’s trichrome, X400
In the LED7 group (Fig. 4a–d), it was found that the epitendon and endotendon had abundant granulated tissue, characterised by fibroblasts and newly formed blood vessels, in addition to the inflammatory mononuclear cells. Compared with the TD7 group (Fig. 2a–d), fibroblasts were more numerous and more organised, especially near the existing bundles. The level of organisation, however, was apparently lower than in the US7 group (Fig. 3a–d). In the LED14 group (Fig. 4e–h), the histological features of this group were, in general, very similar to those observed in the US14 group (Fig. 3e–h). The vessels became quite scarce and the extracellular matrix was dense. Fibroblasts were more slender and spindly with characteristics closer to fibrocytes and bundles oriented parallel to the undamaged fibres.
Photomicrographs indicating histological aspects of the tendon of the animals in the groups LED7 (LED, 7th day (a–d)) and LED14 (LED, 14th day (e–h)). a, b Numerous fibroblasts are arranged in well-defined bundles in the epitendon; inflammatory cells are scarce. c, d Extracellular matrix is loose, with newly formed vessels and fibroblasts. e, f Parallel bundles of newly formed fibroblasts arranged in parallel in the epitendon, blood vessels are scarce. g, h Thin fibroblasts separated by dense extracellular matrix and with a staining pattern similar to that observed in the original tendon; inflammatory cells are not seen and blood vessels are scarce. a, e H & E, X100; b, f Masson’s trichrome, X100; c, g H & E, X400; d, h Masson’s trichrome, X400
Histomorphometric measurements
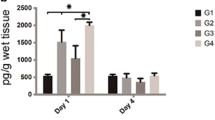
On the 7th day, all experimental groups showed a higher number of fibroblasts compared to the H group (p < 0.05), indicating a repair process under way in all tendinitis-induced groups (Fig. 5). The rats of the US7 and LED7 groups did not present a statistically significant difference in the number of fibroblasts when compared to the TD7 group. On the 14th day, all experimental groups showed a higher number of fibroblasts compared to the H group (p < 0.05), indicating that the repair process is still under way in the tendinitis-induced groups (Fig. 5). The rats of the US14 group presented significantly lower number of fibroblasts when compared to the TD14 group (p < 0.05), which indicates better anti-inflammatory response with the use of US for 14 days.
FT-Raman analysis
Figure 6 shows the average normalised spectra for all groups used in the work. These spectra are dominated by strong bands of structural proteins (mainly collagen type I and III). The most important vibrational bands found in the spectra of tendon tissues are marked with dotted lines; a tentative attribution based on the biochemical constitution of tendons can be found in the literature [26, 28, 29].
By observing the Raman spectra in Fig. 6, it was found that the spectral information is similar in all groups, with the exception of group H, which revealed some spectral characteristics of triolein (adipocytes). Due to the similar spectral characteristic of the collagens presented in the tendon tissues (mainly collagen I and III), it was difficult to visualise such small differences. In order to extract relevant information regarding tissue constitution, a spectral model was developed in order to estimate the contribution of each collagen type in the Raman spectra of each group. Collagen III is synthesised at the beginning of tissue repair, being substituted by collagen I in the maturation phase, being a marker of tissue remodelling.
Figure 7 presents the spectra of collagen I, III and triolein. The main bands of these constituents are in the same positions as the ones found in tendons. Triolein was included in the model due to its presence in some Raman spectra, which could be an indicative of sampling of circumjacent adipose tissue. Some triolein Raman bands are in the same positions as the protein bands, therefore its inclusion would avoid over estimation of proteins by the spectral model.
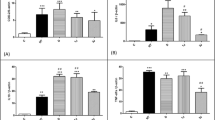
Figure 8 presents the relative Raman concentration of collagen I and collagen III for each experimental group (7th and 14th day). As mentioned in “Methods,” the spectrum of triolein was included in the model to avoid overestimation of collagen due to overlap of some bands of both compounds but not used in the analysis. Raman spectroscopy showed that the relative amount of collagen I and III presented statistically significant differences among the groups on the 7th and 14th day (ANOVA, p < 0.05). Despite not being statistically significant, all experimental groups presented lower amounts of collagen I compared to H on the 7th day (Fig. 8a), and groups TD7 and US7 presented higher amounts of collagen III compared to H, while LED7 had similar amounts compared to H. On the 14th day (Fig. 8b), TD14 and US14 groups presented lower amounts of collagen I compared to H, being US14 significantly lower (p < 0.05), and group LED14 presented higher amounts of collagen I compared to H and significantly higher compared to LED7 (p < 0.05). For collagen III, all experimental groups on the 14th day presented higher amounts of collagen III compared to H. By comparing the 7th and 14th day, US group presented lower amounts of collagen I and higher of collagen III on the 14th day, LED group presented higher amounts of collagen I and collagen III on the 14th day. This result would indicate for LED groups a more abundant and well organized collagen fibres and a better quality of the healing.
Relative Raman concentration of collagen I and collagen III estimated by the biochemical fitting model for the experimental groups: a comparison of the amount of collagen I and b comparison of the amount of collagen III, at 7 and 14 days. Single asterisk and double asterisk indicate columns with statistically significant differences between the 7th and 14th day (ANOVA, p < 0.05, Tukey–Kramer post-test)
Discussion
The process of tissue repair is complex and can be conceptualised as a chain of events, each one completing the previous and beginning the next: hemostasis, inflammation, phagocytosis, proliferation and maturation [32]. The inflammatory phase of the tendinous tissue occurs from the 1st to the 7th day, where macrophages remove cellular debris and change extracellular components and fibroblasts are radially arranged toward the tendon fibres, synthesising collagenous fibres around the 5th day of injury [15].
The proliferation of fibroblasts and the evolution of the inflammatory–reparative process in tendinitis involve well-characterised steps. According to Maia Filho [2], necrosis, haemorrhage and neutrophilic inflammatory exudate were the predominant histological findings on the 1st day, while after the 3rd day, these changes began to decline, with the number of mononuclear inflammatory cells, especially macrophages, increasing. Maia Filho [2] found that there was a significant change in the histological images on the 7th day, with discrete infiltration of peri- and intra-tendinous mononuclear inflammatory cells, intense fibroblast proliferation and moderate neoangiogenesis. At this stage, the authors found tissue with a characteristic granulation. Similarly on the 14th day, production and the reorganisation of collagen fibres had already started [2].
This study showed histological results in the H group similar to those described in the literature [2, 30, 31]. Histological analysis of the tendinitis-induced group revealed that the inflammation is still increasing up to the 7th day, and the proliferative phase occurred on the 14th day. In terms of anti-inflammatory response and tissue repair, measured by the amount of fibroblast cells at the site of lesions, the US therapy showed to be better at 14 days compared to LED therapy. This was evidenced by the reorganisation of collagen fibres and tissue maturation observed in the photomicrographs of lesioned tissues (Figs. 3 and 4). The counting of the number of fibroblasts also revealed a lower level of fibroblast proliferation in the US group, which is indicative of anti-inflammation effect.
Similar results to those obtained in this study showed that the phase of tissue repair is very dynamic, with numerous types of cells, such as platelets, mast cells, macrophages, and neutrophils, entering and leaving the wound site [3]. These studies indicated that therapeutic ultrasound can interact with these cells, influencing their activity and leading to acceleration of the repair, while reducing inflammation [12]. In the proliferative and remodelling phase, ultrasound assists in the reorganisation (arrangement and alignment) of collagen fibres, a phenomenon that is attributed to the piezoelectric transducer [2].
The groups treated with LED showed no statistically significant difference in the number of fibroblasts when compared to the TD7 and TD14 groups. Casalechi et al [31]. showed that therapy with low-power LED enhanced the anti-inflammatory effect and tissue response in inflammatory process in a experimental model of Achilles tendinitis. Xavier et al [30]. suggested that the therapy with low-power LED (880 nm) enhanced the tissue response after 7 and 14 days of treatment in the collagenase-induced tendinitis in rats, reducing the signs of inflammation by reducing the number of inflammatory cells and decrease mRNA expression of cytokines. These contrasting results can be attributed to different responses from different tissues under non-coherent light illumination and to different protocols (energy density per point, total energy and number of doses) used in LED applications, which makes further research on an effective treatment protocol for LED therapy being necessary.
The analysis of the Raman spectra of tendons in the experimental groups did not show relevant spectral differences among them. Only the H group presented, besides the protein features, some features of adipose tissue (triolein), which could be due to some interstitial fat left from the dissection procedure. This fat has been spectrally modelled in order to avoid them causing an overestimation of collagen. The quantitative model revealed that the dynamics of collagen production depends on the treatment to which the tissue was submitted. Collagen III was significantly lower for the LED group compared to the control group on the 7th day, and significantly increased for the LED group after 14 days. Regarding collagen I, the US group showed a lower amount for the 14th day, while the LED group showed a lower amount for the 7th day and statistically significant increase for the 14th day. A decrease of collagen III and increase of collagen I occurs in the maturation phase, where the temporary matrix containing collagen III is slowly substituted by the definitive matrix, which is rich in collagen I. Groups that had an increased amount of collagen III showed a lower amount of collagen I, such as the TD7 and US14. In the LED group, the effect was contrasting, with lower amounts of collagen I and III on the 7th day, compared to TD7 but a higher amount of collagen I and III for the 14th day compared to 7th day. This suggests a strong stimulus to cells to synthesize collagen III and its conversion to a mechanically strong collagen I when tissue was irradiated by the LED. The high standard deviation bars in the Raman quantitative model could indicate non-homogeneous biochemical changes occurring during the remodelling process.
The beneficial effects of adjuvant therapy with US and LED have a lot to contribute in relation to anti-inflammatory effect and collagen synthesis in tendon healing, and the biochemical composition of the remodelling tissue could be measured through Raman spectroscopy, allowing differentiation between treatments in terms of collagen deposition, revealing that LED treatment promoted higher protein synthesis.
Conclusion
The present study demonstrated that the experimental group treated with US for 14 days showed a better anti-inflammatory response than both LED-treated and control groups, measured through histomorphometry and counting of fibroblasts. Raman spectroscopy revealed that the synthesis of collagen I and III was higher for the LED-treated groups compared to both the control and US in 7 and 14 days, indicating a high rate of matrix synthesis and a better quality tissue healing process under way.
References
Maffulli N, Kader D (2002) Tendinopathy of tendo achillis. J Bone Joint Surg 84-B:1–8
Maia Filho ALM, Villaverde AB, Munin E, Aimbire F, Albertini R (2010) Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol 36:1682–1690
Maffulli N, Ewen SWB, Waterston SW, Reaper J, Barras V (2000) Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med 28:499–505
Covizi DZ, Felisbino SL, Gomes L, Pimentel ER, Carvalho HF (2001) Regional adaptations in three rat tendons. Tissue Cell 33:483–490
Aglietti P, Buzzi R, Insall JN (2001) Disorders of patellofemoral joint. In: Insall JN, Scott WN (eds) Surgery of the knee, 3rd edn. Churchill Livingstone, New York, pp 913–1043
Korpan MI, Samosiuk NI, Magomedov OM, Brusko AT, Stezhka VA, Burianov AA, Chekman IS, Fialka-Mozer V (2006) Clinical and experimental aspects of influence of ultrasound on tissue regeneration. Lik Sprava 7:55–61
Habeeb F, Stables G, Bradbury F, Nong S, Cameron P, Plevin R, Ferro VA (2007) The inner gel component of Aloe vera suppresses bacterial induced pro-inflammatory cytokines from human immune cells. Methods 42:388–393
Hamman JH (2008) Composition and applications of Aloe vera leaf gel. Molecules 13:1599–1616
Karatay S, Aygul R, Melikoglu MA, Yildirim K, Ugur M, Erdal A, Akkus S, Senel K (2009) The comparison of phonophoresis, iontophoresis and local steroid injection in carpal tunnel syndrome treatment. Joint Bone Spine 76:719–722
Koeke PU, Parizotto NA, Carrinho PM, Salate AC (2005) Comparative study of the efficacy of the topical application of hydrocortisone, therapeutic ultrasound and phonophoresis on the tissue repair process in rat tendons. Ultrasound Med Biol 31:345–350
O’Brien WD (2007) Ultrasound-biophysics mechanisms. Prog Biophys Mol Biol 93:212–255
Witte RS, Kim K, Martin BJ, O’Donnell M (2006) Effect of fatigue on muscle elasticity in the human forearm using ultrasound strain imaging. Conf Proc IEEE Eng Med Biol Soc 1:4490–4493
Planas J, Cervelli V, Planas G (2001) Five-year experience on ultrasonic treatment of breast contractures. Aesthet Plast Surg 25:89–93
Lake SP, Ansorge HL, Soslowsky LJ (2008) Animal models of tendinopathy. Disabil Rehabil 30:1530–1541
Trelles M, Alonnes I, Mayo E (2006) Er.YAG laser ablation of plantar verrucae with red Led therapy-assisted healing. Photomed Laser Surg 24:494–498
Mavropoulos A, Staudt CB, Kiliriadis S, Krejci I (2005) Light curing time reduction: in vitro evaluation of new intensive light-emitting diode curing units. Eur J Orthod 27:408–412
Yasukawa A, Ohrui H, Koyama Y, Nagay M, Takakuda K (2007) The effect of low reactive-level laser therapy (LLLT) with helium-neon laser on operative wound healing in rat model. J Vet Med Sci 68:799–806
Demir H, Menku P, Kirnap M, Calis M, Ikizceli I (2004) Comparison of the effects of laser, ultrasound, and combined laser + ultrasound treatments in experimental tendon healing. Lasers Surg Med 35:84–89
Penteado SC, Fogazza BP, Carvalho CS, Arisawa EAL, Martins MA, Martin AA, Martinho HS (2008) Diagnosis of degenerative lesions of supraspinatus rotator cuff tendons by Fourier transform- Raman spectroscopy. J Biomed Opt 13:14–18
Wopenka B, Kent A, Jill D, Pasteris YY, Thomopoulos S (2008) The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl Spectrosc 62:1285–1294
Lopes CB, Pacheco MTT, Silveira L, Cangussú MCT, Pinheiro ALB (2010) The effect of the association of near infrared laser therapy, bone morphogenetic proteins and guided bone regeneration on tibial fractures treated with internal rigid fixation: a Raman spectroscopic study. J Biomed Mat Res A 94:1257–1263
Silva FF, Souza RA, Pacheco MTT, Ribeiro W, Silva MASR, Miranda H, Salgado MAC, Castilho JCM, Silveira L (2011) Effects of different swimming exercise intensities on bone tissue composition in mice: a Raman spectroscopy study. Photomed Laser Surg 29:217–225
Ball DW (2001) Rayleigh and Raman scattering. Spectrosc Int J 16:28–31
Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45:R1–R59
de Jong BWD, Schut TCB, Maquelin K, van der Kwast T, Bangma CH, Kok DJ, Puppels GJ (2006) Discrimination between nontumor bladder tissue and tumor by Raman spectroscopy. Anal Chem 78:7761–7769
Bodanese B, Silveira L, Albertini R, Zângaro RA, Pacheco MTT (2010) Differentiating normal and basal cell carcinoma human skin tissues in vitro using dispersive Raman spectroscopy: a comparison between principal components analysis and simplified biochemical models. Photomed Laser Surg 28:119–127
Stone N, Prieto MCH, Crow P, Uff J, Ritchie AW (2007) The use of Raman spectroscopy to provide an estimation of the gross biochemistry associated with urological pathologies. Anal Bioanal Chem 387:1657–1668
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 42:493–541
Stone N, Kendall C, Smith J, Crow P, Barr H (2004) Raman spectroscopy for identification of epithelial cancers. Faraday Discuss 126:141–157
Xavier M, David DR, Souza RA, Arrieiro AN, Miranda H, Santana ET, Silva JA Jr, Salgado MAC, Aimbire F, Albertini R (2010) Anti-inflammatory effects of low-level light emitting diode therapy on Achilles tendinitis in rats. Lasers Surg Med 42:553–558
Casalechi HL, Nicolau RA, Casalechi VL, Silveira L, De Paula AMB, Pacheco MTT (2009) The effects of low-level light emitting diode on the repair process of Achilles tendon therapy in rats. Lasers Med Sci 24:659–665
Carvalho KC, Nicolau RA, Maia Filho ALM, Barja PR, Sá HP, Santo LAE, Rocha GM (2010) Study of the strength of healing skin of rats treated with phototherapy in laser. ConScientiae Saúde 9:179–186. doi:10.5585/conssaude.v9i2.2267
Acknowledgments
L. Silveira, Jr. acknowledges National Counsel of Technological and Scientific Development (CNPq) for the Productivity Fellowship (no. 309209/2011-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moura Júnior, M.J., Arisawa, E.Â.L., Martin, A.A. et al. Effects of low-power LED and therapeutic ultrasound in the tissue healing and inflammation in a tendinitis experimental model in rats. Lasers Med Sci 29, 301–311 (2014). https://doi.org/10.1007/s10103-013-1327-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1327-0