Abstract
The objective of this study was to investigate the effects of two lasers (Er:YAG and CO2) in enhancing skin permeation of three vitamin C derivatives, L-ascorbic acid 2-phosphate sesquimagnesium salt (MAP-1), magnesium L-ascorbic acid-2-phosphate (MAP-2), and 2-phospho-L-ascorbic acid trisodium salt (SAP). Dorsal skin of 1-week-old pathogen-free pigs was used for this in vitro study. Changes in permeation in laser-treated skin treated by the lasers were examined by confocal scanning electron microscopy. Transdermal flux of vitamin C derivatives was examined with a Franz diffusion cell. Fluxes of MAP-1, MAP-2, and SAP across Er:YAG laser-treated skin were 15–27-fold, 48–123-fold, and 22–56-fold higher, respectively, than their fluxes across intact skin. The fluxes of MAP-1, MAP-2, and SAP across CO2 laser-treated skin were 28–36-fold, 116–156-fold, and 79–102-fold higher, respectively, than their fluxes across intact skin. Optimal fluency for the Er:YAG laser was 3.8 J/cm2 for MAP-1 and 5 J/cm2 for MAP-2 and SAP. Optimal fluency for the CO2 laser was 5 W for all three derivatives. In conclusion, optimal fluency for all derivatives was 5 W for the CO2 laser and 3.8 to 5 J/cm2 for the Er:YAG laser.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin C inhibits melanogenesis, promotes collagen synthesis, and has anti-oxidant properties [1–5]. Transdermal delivery of vitamin C, a compound used topically as a skin whitener and anti-aging agent, is difficult because the outer layer of the skin, the stratum corneum, provides a barrier to the entry of water-soluble substances such as vitamin C [6]. A number of manipulations have been tried to remove this impediment, such as tape-stripping to remove the stratum corneum physically, application of an electrical gradient to drive charged molecules across the barrier, or electroporation to open tiny holes in this skin layer [7].
Laser pretreatment is one of the methods used to increase permeability. Two lasers used for this treatment are the erbium-doped aluminum garnet (Er:YAG) laser and the CO2 laser. An energy pulse from either laser can ablate the stratum corneum and at higher fluencies, decrease the thickness of the epidermal layer, and cause changes in both epidermis and dermis, thus increasing transdermal permeability [5]. The two laser types differ in the wavelengths used. The Er:YAG laser has a shorter wavelength and a reduced penetration into tissue. The CO2 laser has a longer wavelength, a much lower absorption coefficient in water, and can cause necrosis due to thermal damage [8–10].
Although the use of vitamin C itself as a skin whitener is limited because it is unstable and easily oxidized and inactivated, three stable vitamin C derivatives, L-ascorbic acid 2-phosphate sesquimagnesium salt (MAP-1), magnesium L-ascorbic acid-2-phosphate (MAP-2), and 2-phospho-L-ascorbic acid trisodium salt (SAP), have been used in skin-whitening products for a number of years [11].
Previous studies of the use of lasers to increase permeability to vitamin C and its derivatives have not identified the maximum (optimal) transdermal energy for each type of laser for each vitamin C derivative. However, it would be useful clinically to identify the optimal laser setting in order to avoid skin damage due to unnecessarily high settings.
In the current study, the effect of and the optimal settings for two lasers (Er:YAG and CO2) in increasing transdermal permeability for each of the above three stable vitamin C derivatives were examined in vitro in porcine skin.
Materials and methods
Materials
L-ascorbic acid 2-phosphate sesquimagnesium salt (MAP-1, 289.54) and 2-phospho-L-ascorbic acid trisodium salt (SAP, 322.05) were obtained from Sigma (St. Louis, MO, USA). Magnesium L-ascorbic acid-2-phosphate (MAP-2, 759.22) was obtained from SHOWA DENKO K.K. Company (Japan).
Laser experimental protocol
Er:YAG (2,940 nm) and CO2 (10,600 nm) lasers were used to pretreat samples taken from the dorsal region of porcine skin. The Er:YAG laser (Profile-MP, Sciton Inc., Palo Alto, CA. USA) was used at output energies of 0.31~0.79 J/pulse with a beam spot size of 4 mm, a setting that achieved fluencies of 2.5~6.3 J/cm2. One pass of the CO2 laser (150XJ, Sharplan Laser Inc., Israel) with a pulse of 5~11 W and a beam diameter of 5 mm was also used.
Skin samples
Skin from 8 pathogen-free pigs (1 week old) was supplied by the Animal Technology Institute Taiwan (Miaoli, Taiwan). The supplier euthanized the piglets by electrocution, harvested the dorsal skin, cut it to the appropriate size, and shipped it directly to the author’s laboratory. The resulting skin samples were used for scanning electron microscopy (SEM), confocal scanning electron microscopy (CLSM), and in vitro permeation studies of the three vitamin C derivatives.
Ultrastructure examination by scanning electron microscopy
Excised porcine skin samples with and without laser treatment were cut into appropriate-sized cubes and immediately fixed at 4 °C in 3 % paraformaldehyde and 2 % glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) overnight, washed three times with 0.1 M cacodylate and 7 % sucrose buffer for 15 min, post-fixed with 2 % osmium tetroxide for 1 h, washed three times as before, and immersed in 0.5 % aqueous uranyl acetate for 30 min. The specimens were then dehydrated in graded concentrations of ethanol, transferred to isoamyl acetate, and critical-point dried using liquid CO2. The dried specimens were affixed with gold-palladium in an ion coater and examined with a scanning electron microscope (Hitachi S-5000, Japan).
In vitro permeation of vitamin C derivatives
For permeation studies, a piece of excised porcine dorsal skin was mounted in a Franz diffusion cell with the stratum corneum facing the donor compartment. After laser pretreatment at defined fluencies, the skin surface was wiped with a cotton wool swab several times. The receptor compartment (5.5 ml) was filled with a pH 7.4 citrate–phosphate buffer. The donor compartment (0.5 ml) contained 13 mM MAP-1, MAP-2, or SAP in a pH 7.4 citrate–phosphate buffer. The available area of the side-by-side cell was 0.7854 cm2. The receptor compartment was maintained at 37 °C, and the contents were stirred with a magnetic bar at 600 rpm. At appropriate intervals, 300 μl aliquots of receptor medium were withdrawn and immediately replaced with an equal volume of fresh receptor solution. The length of the sampling period was 12 h. The amount of drug in the receptor medium was determined by high-performance liquid chromatography (HPLC).
HPLC analytical method
The vitamin C derivative content in the various samples was analyzed with an HPLC system consisting of a Dionex UltiMate 3000 pump, a Dinoex UltiMate 3000 autosampler, and a Dinoex UltiMate 3000 UV detector. A 15-cm-long, 4.6-mm-inner diameter Inertsil ODS-4V column (GL Science, Tokyo, Japan) was used. For MAP-1, MAP-2, and SAP, the mobile phase was used at a flow rate of 1 ml/min, and the UV detector was set at a wavelength of 254 nm.
In vitro permeation of fluorescein isothiocyanate examined through confocal laser scanning microscopy
Skin was first treated by irradiation with a Er:YAG (5 J/cm2) or CO2 (5 W) laser. Fluorescein isothiocyanate (FITC)/pH 7.4 buffer (0.2 ml) at a concentration of 0.2 % was added to the donor compartment for a 2-h incubation. All procedures were carried out in the dark to prevent interference by ambient light. Skin samples obtained were then examined for FITC fluorescence by CLSM. The skin thickness was optically scanned through the Z-axis of a Leica TCS SP2 confocal microscope (Manheim, Germany) at about 8-μm increments. Optical excitation was carried out with a 488-nm argon laser beam and the fluorescence emission was detected at 590 nm.
Data analysis
For the in vitro permeation study, the total amount of drug permeating across a unit diffusion surface was calculated and plotted as a function of time. The flux was calculated from the slope of the linear portion of the cumulative amount/time plot and expressed as nmole/cm2/h.
Two pulses at different sites of the 4-mm-diameter hand-piece of the Er:YAG laser covered 32 % of the total skin area available for permeation. One pulse of the 5-mm-diameter hand-piece of the CO2 laser covered 25 % of this area. We calculated normalized flux by extrapolating the laser-increased permeation that was recorded to 100 % of the total area using the following algorithm:

- J100% :
-
Flux (nmole/cm2/h) across 100 % laser-treated skin (normalized flux)
- Jlaser :
-
Flux across partly laser-treated skin in Franz cell (original flux)
- Jcontrol :
-
Flux across untreated skin
- Atotal :
-
Area (cm2) of total diffusion area
- Alaser :
-
Area of laser-treated site
Statistical analysis
Cumulative amount–time profiles for in vitro transdermal permeation were graphed as a line plot with mean and standard deviation (SD) for each condition. Results of normalized flux across the porcine skin were summarized as mean ± SD for each condition and were compared using one-way ANOVA with a post hoc Duncan test. Statistical assessments were two-sided and considered significant when P < 0.05. An adjusted significant level P = 0.01 (=0.05/5) was used for post-hoc multiple comparisons. Data were graphed and analyzed using SigmaPlot software (Systat Software, Inc).
Results
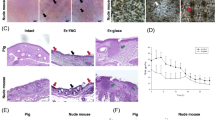
Confocal microscopy of FITC penetration of porcine skin after Er:YAG and CO2 laser treatment is shown in Fig. 1. The upper section of each panel shows optical scanning of a series of 8 μm increases in depth below the skin surface. The single section at the bottom of the panel is a compilation of the results at all depths. These micrographs show that pretreatment by either laser increases the depth to which FITC can penetrate into the skin layer. The CO2 laser (5 W) causes more extensive penetration than the Er:YAG laser (5 J/cm2).
Confocal laser scanning microscopic (CLSM) micrographs of porcine skin after in vitro topical administration of FITC after laser pretreatment: a Non-treatment group. b Er:YAG laser, 5 J/cm2. c CO2 laser, 5 W (original magnification, ×20). The skin specimen was viewed by CLSM at 8-μm increments through the Z-axis. The images below the photographs of the 15 fragments are the sum of all fragments
Figure 2 shows SEM images of porcine skin after exposure to Er:YAG or CO2 laser treatment. In untreated skin (panel A), one can see an orderly pattern of overlapping corneocytes. After Er:YAG treatment (panel B) order is lost, and spaces between the corneocytes and some ablation are present. CO2 laser treatment produces more severe disruption of the corneocyte barrier, hills and valleys in the skin surface, and greater spaces between the corneocytes.
Figures 3 and 4 show the effect of Er:YAG laser pretreatment. Figure 3 shows cumulative amount/time for transdermal permeation of MAP-1, MAP-2, and SAP at different Er:YAG fluencies. When fluency increased, the permeation rate increased for all three compounds. The maximum cumulative amount at the highest fluency used was approximately 700 nmole/cm2 for MAP-1 (Fig. 3a), 900 nmole/cm2 for MAP-2 (Fig. 3b), and 700 nmole/cm2 for SAP (Fig. 3c).
MAP-1, MAP-2, and SAP normalized fluxes across porcine skin after pretreatment with an Er:YAG laser (n = 8 for each fluency condition). Each value represents the means ± SD. Normalized flux was calculated from the flux across fully laser-treated skin (100 %). P < 0.05 through one-way ANOVA test with a post hoc Duncan test. Difference letters (a, b, c, d) indicate significantly different between groups
Normalized fluxes for the three derivatives are shown in Fig. 4. These data suggest that a dose of 3.8 J/cm2 might be the optimal fluency use to increase permeation for MAP-1, and 5 J/cm2 might be the optimal fluency for both MAP-2 and SAP.
Figures 5 and 6 show the effect of CO2 laser pretreatment. Figure 5 shows cumulative amount/time for transdermal permeation of MAP-1, MAP-2, and SAP for different intensities of CO2 laser pretreatment. As with Er:YAG laser pretreatment, as the intensity of the laser stimulus increased, the permeation rate increased for all three compounds. The maximum cumulative amount at the highest fluency used is approximately 800 nmole/cm2 for MAP-1 (Fig. 5a), 900 nmole/cm2 for MAP-2 (Fig. 5b), and 1,200 nmole/cm2 for SAP (Fig. 5c).
MAP-1, MAP-2, and SAP normalized fluxes across porcine skin after pretreatment with CO2 laser (n = 8 for each formulation condition). Each value represents mean ± SD. Normalized flux was calculated from the flux across fully laser-treated skin (100 %). P < 0.05 through one-way ANOVA test with a post hoc Duncan test. Difference letters (a, b) indicate significantly different between groups
Figure 6 shows normalized fluxes for the three derivatives under the four CO2 laser stimulus intensities used. The results suggest that a pulse of 5 W might be the optimal intensity to use for all three derivatives to enhance permeation.
Discussion
Both Er:YAG and CO2 lasers increased permeability to all three vitamin C derivatives. At 5 J/cm2 (Er:YAG laser) and 5 W (CO2 laser), the CO2 laser increased permeability to each compound more than the Er:YAG laser and showed deeper penetration of the fluorescent probe, FITC, into the skin layer. Maximum (optimal) transdermal delivery energy was 3.8–5 J/cm2 (Er:YAG laser) and 5 W (CO2 laser).
Hsiao et al. [12] have compared the effect of the two lasers on permeation of two other stable vitamin C derivatives, 3-O-ethyl ascorbic acid and 1-ascorbyl-2-glucoside, in nude mouse skin. As fluency was increased a ceiling effect on permeation was seen with the Er:YAG laser, but not with the CO2 laser. The optimal fluency for the two compounds tested was 5 J/cm2. Optimal fluency for the CO2 laser was not reported. Lee et al. [5] found the Er:YAG laser able to increase permeability to vitamin C, but not to magnesium ascorbyl phosphate. They did not investigate the ability of the CO2 laser to increase permeability to this derivative.
The three derivatives studied in the current investigation are used in a number of topical formulations for skin whitening. When laser pretreatment is used in order to enable the derivatives to cross the stratum corneum, it is important for the clinician to know the optimal laser setting in order to avoid skin damage due to high energy. The Er:YAG laser and the CO2 laser require different machines. Not all clinics have both machines, so it is important to know the best settings for each type of laser.
The primary barrier to transdermal drug delivery is the low permeability of the stratum corneum. Laser treatment can be divided into two components, a stratum corneum ablation effect and the effect of photochemical waves. Of the two components, the stratum corneum ablation effect is the most important [13], and laser treatment can remove the stratum corneum with little damage to the other layers of the skin [14]. This ability has enabled laser treatment to be used for such applications as transdermal DNA delivery [15] and to immunize an animal without the addition of adjuvants or penetration enhancers [16].
Other ways to increase the effectiveness of Er:YAG laser pretreatment while decreasing potential skin damage have been investigated. In fractional treatment [16–20], the Er:YAG laser beam was sent through a grid with small holes in it so that only a fraction of the treated area was reached by the beam. In microporation [7], the Er:YAG laser, whose wavelength is the principal wavelength for the absorption of water molecules, was used with a very short pulse duration in pig and human skin samples. Heat transfer to other substances was thus minimized and the explosive evaporation of the water produced micropores through which the drug could enter the skin. And Greene et al. [8], in a clinical study, has explored the use of blended Er:YAG and CO2 laser treatment in patients with actinic skin damage.
In considering the clinical use of CO2 laser treatment to increase transdermal delivery of vitamin C, one question is whether hyperpigmentation, a known side effect of CO2 laser treatment, would interfere with results. Two studies of long-term side effects of CO2 laser resurfacing reported hyperpigmentation in 3/104 (2.8 %) and 7/104 (6.3 %) of patients [21,22]. No instances of melasma were reported, and hyperpigmentation was treated with topical hydroquinone. But the goals of CO2 laser treatment for vitamin C delivery are different from those for skin resurfacing. To increase skin permeability, the goal is to remove the stratum corneum, and only the lowest laser setting, 5 W, was needed in our study. In skin resurfacing, deeper skin layers need to be removed, and in the Bernstein study [21], for example, 1 to 4 passes at 10–20 W were used. It seems likely, therefore, that hyperpigmentation will not be a problem with combined CO2 laser/vitamin C use.
We have shown in this study that pretreatment with both the Er:YAG and CO2 laser increase the permeability of porcine skin to three stable vitamin C derivatives used in topical skin care formulations. Optimal fluency for all derivatives was 5 W for the CO2 laser and 3.8 to 5 J/cm2 for the Er:YAG laser.
References
Reszko AE, Bereson D, Lupo MP (2009) Cosmeceuticals: practical applications. Dermatol Clin 27:401–416
Humbert PG, Haftek M, Creidi P, Lapière C, Nusgens B, Richard A, Schmitt D, Rougier A, Zahouani H (2003) Topical ascorbic acid on photoaged skin. Clinical topographical and ultrastructural evaluation: double-blind study vs. placebo. Exp Dermatol 12:237–244
Traikovich SS (1999) Use of topical ascorbic acid and its effects on photodamaged skin topography. Arch Otolaryngol Head Neck Surg 125:1091–1098
Espinal-Perez LE, Moncada B, Castanedo-Cazares JP (2004) A double-blind randomized trial of 5 % ascorbic acid vs. 4 % hydroquinone in melasma. Int J Dermatol 43:604–607
Lee WR, Shen SC, Wang KH, Hu CH, Fang JY (2003) Lasers and microdermabrasion enhance and control topical delivery of vitamin C. J Invest Dermatol 121:1118–1125
Lee WR, Shen SC, Lai HH, Hu CH, Fang JY (2001) Transdermal drug delivery enhanced and controlled by erbium:YAG laser: a comparative study of lipophilic and hydrophilic drugs. J Control Rel 75:155–166
Bachhav YG, Summer S, Heinrich A, Bragagna T, Böhler C, Kalia YN (2010) Effect of controlled laser microporation on drug transport kinetics into and across the skin. J Control Rel 146:31–36
Greene D, Egbert BM, Utley DS, Koch RJ (2000) In vivo model of histologic changes after treatment with the superpulsed CO2 laser, erbium:YAG laser, and blended lasers: a 4- to 6- month prospective histologic and clinical study. Lasers Surg Med 27:362–372
Lee WR, Shen SC, Wang KH, Hu CH, Fang JY (2002) The effect of laser treatment on skin to enhance and control transdermal delivery of 5-fluorouracil. J Pharm Sci 91:1613–1626
Alexiades-Armenakas MR, Dover JS, Arndt KA (2008) The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol 58:719–737
Balaguer A, Chisvert A, Salvador A (2008) Environmentally friendly LC for the simultaneous determination of ascorbic acid and its derivatives in skin-whitening cosmetics. J Sep Sci 31:229–236
Hsiao CY, Huang CH, Hu S, Ko YS, Sung HC, Huang SY (2011) Skin pretreatment with lasers promotes the transdermal delivery of vitamin C derivatives. Lasers Med Sci 26:369–376
Lee WR, Shen SC, Fang CL, Liu CR, Fang JY (2007) Skin pretreatment with an Er:YAG laser promotes the transdermal delivery of three narcotic analgesics. Lasers Med Sci 22:271–278
Shen SC, Lee WR, Fang YP, Hu CH, Fang JY (2006) In vitro percutaneous absorption and in vivo protoporphyrin IX accumulation in skin and tumors after topical 5-aminolevulinic acid application with enhancement using an erbium:YAG laser. J Pharm Sci 95:929–938
Lee WR, Shen SC, Liu CR, Fang CL, Hu CH, Fang JY (2006) Erbium:YAG laser-mediated oligonucleotide and DNA delivery via the skin: an animal study. J Control Rel 115:344–353
Lee WR, Pan TL, Wang PW, Zhuo RZ, Huang CM, Fang JY (2008) Erbium:YAG laser enhances transdermal peptide delivery and skin vaccination. J Control Rel 128:200–208
Forster B, Klein A, Szeimies RM, Maisch T (2010) Penetration enhancement of two topical 5-aminolaevulinic acid formulations for photodynamic therapy by erbium:YAG laser ablation of the stratum corneum: continuous versus fractional ablation. Exper Dermatol 19:806–812
Lee WR, Shen SC, Al-Suwayeh SA, Yang HH, Yuan CY, Fang JY (2011) Laser-assisted topical drug delivery by using a low-fluence fractional laser: imiquimod and macromolecules. J Control Rel 153:240–248
Lee WR, Shen SC, Pai MH, Yang HH, Yuan C, Fang JY (2010) Fractional laser as a tool to enhance the skin permeation of 5-aminolevulinic acid with minimal skin disruption: a comparison with conventional erbium:YAG laser. J Control Rel 145:124–133
Zhang LW, Fang YP, Fang JY (2011) Enhancement techniques for improving 5-aminolevulinic acid delivery through the skin. Dermatol Sin 29:1–7
Bernstein LJ, Kauvar AN, Grossman MC, Geronemus RG (1977) The short- and long-term side effects of carbon dioxide laser resurfacing. Dermatol Surg 23:519–525
Manuskiatti W, Fitzpatrick RE, Goldman MP (1999) Long-term effectiveness and side effects of carbon dioxide laser resurfacing for photoaged facial skin. J Am Acad Dermatol 40:401–411
Acknowledgments
This work was supported by the Chang Gung Memorial Hospital Grant (CMRPF190101).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, CH., Sung, HC., Hsiao, CY. et al. Transdermal delivery of three vitamin C derivatives by Er:YAG and carbon dioxide laser pretreatment. Lasers Med Sci 28, 807–814 (2013). https://doi.org/10.1007/s10103-012-1151-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1151-y