Abstract
The aim of this study was to histologically and histometrically evaluate the influence of repeated adjunctive antimicrobial photodynamic therapy (aPDT) on bone loss (BL) in furcation areas in rats. Periodontitis was induced by placing a ligature around the mandibular molar in 75 rats. The animals were divided into five groups: the SS group was treated with saline solution (SS); the SRP group received scaling and root planing (SRP); the aPDT1 group received SRP as well as toluidine blue (TBO) and low-level laser therapy (LLLT; InGaAlP, 660 nm; 4.94 J/cm2/point) postoperatively at 0 h; the aPDT2 group received SRP as well as TBO and LLLT postoperatively at 0, 24, 28, and 72 h; and the aPDT3 group received SRP, TBO, and LLLT postoperatively at 0, 48, 96, and 144 h. The area of BL in the furcation region of the molar was histometrically analyzed. Data were analyzed statistically (P < 0.05). Animals treated with a single episode of aPDT showed less BL at days 7 and 30 than those who received only SRP treatment. No significant differences were found among the aPDT groups (P > 0.05). Repeated aPDT did not improve BL reduction when compared to a single episode of aPDT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial products released by the periodontopathogenic microorganisms can cause an inflammatory response by the periodontal tissue. Inflammation and destruction of periodontal tissues are largely considered to result from the response of a susceptible host to a microbial biofilm containing bacterial pathogens [1].
The aim of periodontal treatment is to reestablish the biocompatibility of radicular surfaces with disease exposure by removing periodontopathogenic microorganisms, thus allowing for the subsequent attachment of periodontal tissues [2]. Most commonly, this can be achieved by mechanical scaling and root planing (SRP) [3]. However, such treatment is not always successful because it is often difficult to reduce bacterial deposits and their endotoxins from deeper areas of periodontal pockets. Alternative mechanisms of antimicrobial therapy, such as local and systemic antibiotics, have been proposed to address these limitations [4–6]. Also, many bacterial strains have gained resistance to antimicrobial disinfectants, which are often used in parallel to mechanical instrumentation. A new approach to periodontitis treatment, called antimicrobial photodynamic therapy (aPDT), involves the use of low-intensity light and a nontoxic photosensitizer agent [7] to reduce periodontal pathogens. The major advantages of aPDT include its being a specific therapy-targeting cell, having no collateral effects, and which use will not lead to the selection of resistant bacterial species [8, 9]. During aPDT, reactive oxygen is produced to kill target cells when the photosensitizer is excited by light in the presence of oxygen [8, 9].
Past animals studies have found that aPDT successfully controlled the progression of experimental periodontitis using a single application of aPDT [10–14]. Recently, clinical studies in humans have found a reduction in the total number of viable bacteria in periodontal pockets after application of aPDT [15–17]. Other studies have failed to show any additional clinical benefits when aPDT was used in addition to SRP [15, 16]. These clinical studies [15, 16, 18–20] evaluated the effects of using a single application of aPDT on periodontitis. However, Lulic et al. [21] demonstrated that repeated applications (e.g., five times in 2 weeks) of aPDT along with debridement improved clinical results in the residual pockets of patients under periodontal maintenance, with the best effects observed after 6 months. Furthermore, some studies have demonstrated that low-level laser therapy (LLLT) can stimulate fibroblast proliferation with the correct combination of exposition and density parameters [22–24]. With high densities of energy, however, no effects [25, 26] or even a reduction in cellular proliferation has been reported [24, 27]. Nevertheless, Karu [28] suggested that there was a “window of specificity” with a given wavelength and energy density in which the positive effects of LLLT can be achieved. In order to obtain an appropriate biological response, it is necessary to deliver a sufficient number of irradiations at an optimal dose at the correct wavelength. These conflicting results regarding the effects of aPDT on clinical parameters may reflect the lack of a treatment protocol that specifies the number of aPDT applications as well as wavelengths, laser application parameters, drug permanence times, and photosensitizer drug concentrations [5].
Because of the need for a standard protocol regarding the clinical use of aPDT, the aim of the present study was to histologically and histometrically evaluate the influence of repeated episodes of aPDT when treatment is used in addition to conventional scaling and root planing. This study examined the use of aPDT on experimentally induced periodontitis in rats.
Material and methods
Animals
This study was conducted on 75 adult male Wistar rats (250 to 300 g) aged 3 months. The animals were kept in plastic cages with access to food and water ad libitum. Prior to the surgical procedures, all animals were allowed to acclimatize to the laboratory environment for a period of 5 days. All protocols were approved by the Institutional Animal Care and Use Committee of Araçatuba Dental School, São Paulo State University, Araçatuba, SP, Brazil (no. 2008/008466).
Experimental design
Experimental periodontitis protocol
General anesthesia was induced by administering ketamine (0.4 ml/kg) and xylazine (0.2 ml/kg) via intramuscular injection. One mandibular left first molar in each animal was selected to receive a cotton ligature in the submarginal position to induce experimental periodontitis [14]. After 7 days of experimental periodontitis induction, the mandibular ligature was removed from the left first molar of all animals. A computer-generated table was used to randomly divide the 75 rats into five groups. For better standardization, animal 1 was the first choice, followed by animals 2 and 3. The five groups of 15 animals each were defined as follows: (1) saline solution (SS)—the mandibular left molars were submitted to irrigation with 1 ml of saline solution; (2) SRP (scaling and root planing)–the mandibular left molars were submitted to SRP followed by irrigation with 1 ml of saline solution; (3) aPDT1—the mandibular left molars were submitted to SRP and irrigation with 1 ml of phenotiazinium dye (TBO, 100 μg/ml) (Sigma Chemical Co., St. Louis, MO, USA) and, after 1 min, followed by irradiation with LLLT at 0 h (after the ligature removed); (4) aPDT2—the mandibular left molars were submitted to SRP and irrigation with 1 ml of TBO solution (100 μg/ml) and after 1 min, followed by the application of LLLT at 0, 24, 48, and 72 h postoperatively; and (5) aPDT3—the mandibular left molars were submitted to SRP and irrigation with 1 ml of TBO solution (100 μg/ml) and, after 1 min, followed by LLLT at 0, 48, 96, and 144 h postoperatively.
SRP treatment
The left molars of animals in the SRP, aPDT1, aPDT2, and aPDT3 groups were subjected to SRP with manual #1–2 micro mini five Gracey curettes (Hu-Friedy Co. Inc., Chicago, IL, USA) through ten distal–mesial traction movements in both buccal and lingual aspects at 0 h. The furcation and interproximal areas were scaled with the same curettes through cervical–occlusal traction movements. The entire SRP procedure was performed by the same experienced operator (ECGJ).
aPDT treatment
This study used an Indium–Gallium–Aluminum–Phosphorus (InGaAlP) (TheraLase®, DMC Equipments Ltd., São Carlos, São Paulo, Brazil) low-intensity laser with a wavelength of 660 nm and a spot size of 0.0283 cm2. After 1 min of TBO application, LLLT was applied to three equidistant points at each buccal and lingual aspect of the mandibular first molar in contact with the tissue. The therapeutic laser was released with a power level of 0.035 W for 4 s/point, an energy density of 4.94 J/cm2/point (29.64 J/cm2), and an energy of 0.14 J/point. In total, the area received 0.84 J of energy (6 points). Saline solution and TBO were slowly poured into the periodontal pocket using a syringe (1 ml) and an insulin needle (13 mm × 0.45 mm; Becton Dickinson Ind. Ltd., Curitiba, PR, Brazil) without a bevel.
Experimental periods
Five animals from each group were sacrificed at 7, 15, and 30 days after the periodontitis treatment by the administration of a lethal dose of thiopental (150 mg/kg) (Cristália, Ltd., Itapira, SP, Brazil). Their jaws were removed and fixed in 10 % neutral formalin for 48 h.
Laboratory procedures
Following 48 h of fixation in 10 % neutral formalin, the specimens were demineralized in a solution of 18 % ethylenediamine tetraacetic acid (EDTA) for 8 weeks. Paraffin serial sections (4 μm) were obtained in the mesial–distal direction and dyed with hematoxylin and eosin (HE). The sections dyed with HE were analyzed under light microscopy (AxioStar Plus, Carl Zeiss MicroImaging GmbH, 37030 Gottingen, Germany) at 40× magnification to establish the bone loss (BL) level and to characterize the periodontal ligamentation of the furcation region of each first molar [13, 14].
HE staining was used to assess interradicular bone levels at 12.5× magnification. After excluding the first and last sections where the furcation region was evident, five equidistant sections of each specimen block were selected and imaged by a digital camera coupled to a light microscope [13]. The area of BL in the furcation region was determined histometrically using an image analysis system (ImageLab 2000 Software, Diracon Bio Informática Ltd., Vargem Grande do Sul, SP, Brazil). A blinded trained examiner (LAF) selected the sections for histological and histometric analyses. Another blinded calibrated examiner (ML) conducted the data analysis. The values for each specimen were measured three times by the same examiner on different days to reduce variations in the data [13]. The mean values were averaged and statistically compared.
Intraexaminer reproducibility
Before the histometric analyses were performed, the examiner underwent training and completed double measurements of 20 specimens, with a 1-week interval between each measurement. Paired t tests were completed, and no differences were observed in the mean values for comparison (P > 0.05). Additionally, Pearson’s correlation coefficient revealed a very high correlation (0.99, p = 0.000) between the two measurements for both of the histometric analyses.
Statistical analysis
The hypothesis that there were no differences in BL rates in the furcation region between groups was tested using Bioestat 3.0 software (Bioestat, Windows 1995, Sonopress Brazilian Industry, Manaus, AM, Brazil). After normality testing, the histometric data were analyzed using the Shapiro–Wilk test, and the intragroup and intergroup analyses were performed with a two-way analysis of variance (ANOVA; P < 0.05). When ANOVA detected a statistical difference, multiple comparisons were performed with Tukey’s test (P < 0.05).
Results
Histological assessment
Saline solution (SS)
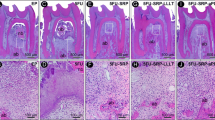
At day 7 post periodontitis treatment, the animals in the SS group presented a degenerative process that was demonstrated by connective tissue with a high number of polymorphonuclear neutrophils and a small quantity of poorly organized bone trabeculae. At days 15 and 30 postperiodontitis treatment (Fig. 1), the connective tissue displayed a small number of fibroblasts and bone tissue, with thin bone trabeculae in the areas of coronal furcation and resorption.
a Photomicrograph illustrating the areas of bone loss in the furcation region of the mandibular left first molar with induced periodontitis in the SS group at 30 days—apical third to the furcation region. b Note the connective tissue displayed a small number of fibroblasts and bone tissue, with thin bone trabeculae in the areas of coronal furcation and resorption (HE; original magnification: a, ×12.5; b, ×40)
Scaling and root planing (SRP)
At days 7 and 30 post periodontitis treatment (Fig. 2), in the SRP group, the presence of well-developed connective tissue with a moderate number of fibroblasts and some blood vessels as well as developed bone tissue and a periodontal ligature with integrity were noted. At 15 days, the connective tissue exhibited a moderate number of fibroblasts as well as discreet vascularization and bone tissue with areas of resorption.
a Photomicrograph illustrating the areas of bone loss in the furcation region of the mandibular left first molar with induced periodontitis in the SRP group at 30 days—middle third to the furcation region. b Note the presence of well-developed connective tissue with a moderate number of fibroblasts and some blood vessels as well as developed bone tissue and a periodontal ligature with integrity (HE, original magnification: a, ×12.5; b, ×40)
aPDT (aPDT1, aPDT2, and aPDT3)
Histological findings from the aPDT1, aPDT2, and aPDT3 groups were similar across all experimental periods. It was possible to observe a small area of well-developed connective tissue with a moderate number of fibroblasts and discreet vascularization. Cementum and dentin areas showed integrity in almost all specimens in the aPDT2 and aPDT3 groups. Bone tissue was characterized by thick and well-differentiated bone trabeculae in all interradicular extensions at 30 days (Figs. 3, 4 and 5).
a Photomicrograph illustrating the areas of bone loss in the furcation region of the mandibular left first molar with induced periodontitis in the aPDT1 group at 30 days—middle third to the furcation region. b Note a small area of well-developed connective tissue with a moderate number of fibroblasts and discreet vascularization (HE, original magnification: a, ×12.5; b, ×40)
a Photomicrograph illustrating the areas of bone loss in the furcation region of the mandibular left first molar with induced periodontitis in the aPDT2 group at 30 days—middle third to the furcation region. b Note a small area of well-developed connective tissue with a moderate number of fibroblasts and discreet vascularization, and cementum and dentin areas showing integrity in almost all specimens (HE; original magnification: a, ×12.5; b, ×40)
a Photomicrograph illustrating the areas of bone loss in the furcation region of the mandibular left first molar with induced periodontal disease in the aPDT3 group at 30 days—middle third to the furcation region. b Note cementum and dentin areas showed integrity in almost all specimens, and bone tissue was characterized by thick and well-differentiated bone trabeculae in all interradicular extensions (HE; original magnification: a, ×12.5; b, ×40)
Histometric assessment
Histometric data are shown in Table 1. Animals in the SS group showed greater BL; a statistically significant difference (P < 0.05) was evident when the SS group was compared to the aPDT1, aPDT2, and aPDT3 groups at days 7, 15, and 30 postperiodontitis treatment (P < 0.05). The histometric results also demonstrated that there was less BL in the aPDT2 and aPDT3 groups than in the SRP group across all experimental periods; these differences were statistically significant (P < 0.05). There was less BL in the aPDT1 group than in the SRP group at days 7 and 30 (P < 0.05).
Discussion
The aim of this study was to examine the effects of repeated episodes of aPDT as an adjunctive treatment of induced periodontitis in rats. In the present study, the induced periodontitis was characterized by clinical signs of gingival inflammation including edema, redness, and attachment loss of gingival tissues.
Photodynamic therapy (PDT) involves the association of a photosensitizing agent with a light source. Studies have shown favorable results using PDT principles against microorganisms involved in periodontitis [6, 10, 29, 30] and periimplantitis [31], and this therapy has been recognized as aPDT [32].
The present study found that animals that received no other treatment except for irrigation with a saline solution (SS) showed greater BL at the furcation region and the presence of poorly organized trabeculae when compared to animals that received SRP treatment. This was true at 7, 15, and 30 days posttreatment, whether or not the SRP treatment was associated with aPDT.
The inflammatory process due to plaque-induced periodontitis causes the formation of inflammatory infiltrate. This infiltrate is initially composed of lymphocytes and macrophages, which both synthesize inflammatory cytokines [33]. Bacterial plaque removal through SRP treatment probably reduced these etiologic factors, providing biocompatibility to the root surface and enabling new periodontal attachments [34]. This improvement was present whether or not SRP was associated with aPDT. However, animals treated with SRP alone clearly displayed more BL than those in the aPDT2 and aPDT3 groups. In addition, there was less BL in the aPDT1 group than in the SRP group at 7 and 30 days posttreatment (P < 0.05). These results confirm the findings of other experimental studies of induced periodontitis in rats that evaluated a single episode of aPDT either alone or in association with SRP [11–14].
In the present study, aPDT associated with SRP may have aided in the removal of polysaccharides present in the extracellular matrices of oral biofilm. These polysaccharides are highly sensitive to the singlet oxygen produced by aPDT. As such, treatment could interrupt bacterial colonization [35].
The beneficial effect of aPDT as a complement to the conventional mechanical treatment of periodontitis in rats was likely a result of its photodestructive effects on various periodontal pathogenic species. These effects could be mediated by a type I reaction (i.e., initiated by superoxide, anionic hydroxyl, or free radicals) or by a type II reaction (i.e., initiated by a singlet oxygen) [32]. These reactive oxygen species are responsible for irreversible damage to bacterial cytoplasmic membranes including protein modification, respiratory chain changes, and nucleic acid alterations [32].
At the onset of periodontitis, venous stagnation as well as a reduction in the oxygen consumption of the tissue occur, which can enhance the growth of anaerobic species [36]. Treatment with aPDT can improve blood flow into the microcirculatory system to reduce venous congestion and increase the oxygenation of gingival tissues. This oxygenation could accelerate the release and use of oxygen, preventing the growth of these microorganisms [36].
aPDT can also have a biostimulating effect on the repair process, accelerating healing by anticipating the change from exudative to proliferative phases, especially in the initial periods, and by increasing collagen deposition [37]. These effects can be attributed to the action of LLLT, which has the advantage of promoting biomodulation in the tissue to be repaired and, therefore, reducing inflammation. LLLT increases mitochondrial respiratory chain and adenosine triphosphate (ATP) synthesis, favors the repair process, induces cell proliferation, promotes the production of nucleic acid, and increases both cell division and collagen synthesis [38]. Some of the effects of laser therapy may be related to an increase in the microcirculation of the irradiated area [39].
In the present study, a comparison of different aPDT treatment protocols revealed that repeated applications of aPDT did not influence the reduction in alveolar BL.
aPDT involves three components: photosensitizer, light, and oxygen [40]. The photosensitizer must reach a high quantic performance of the singlet oxygen and a high lifetime of the triplet state; triplet oxygen production is more effective under these conditions [41]. Photosensitizer activation is dependent on total light doses as well as their penetration depths, dose concentrations, and the location of the target area [42].
To obtain an adequate biological response from tissues irradiated with a low-level laser, it is necessary to utilize the optimal dose of radiation, correct wavelength, and sufficient number of irradiations. The following parameters must be observed: choice of wavelength, energy density, irradiance, operation regimen of the laser, and number of irradiations.
Some clinical studies using a single application of aPDT after SRP treatment did not show significant reductions in clinical inflammatory signs in patients with chronic periodontitis when compared to SRP alone [15, 16, 20]. However, contradictory results were found in another study [21], a randomized controlled clinical trial with ten patients at a 6-month follow-up. The authors evaluated the effects of five episodes of aPDT after initial treatment with SRP (0 h and 1, 2, 7, and 14 days) on residual periodontal pockets with a depth ≥5 mm. They found that those sites treated with aPDT (670 nm, 75 mW/cm2) showed greater reduction in bleeding on probing and probing depths as well as greater clinical attachment level gains.
One relevant characteristic of aPDT action is the optical property of the target tissue. The distribution of LLLT has been shown to be different between normal and pathologic tissues [43]. Cells in a reduced state respond better to laser irradiation [44, 45]. Irradiation with red and infrared light accelerates cellular metabolic processes and can activate proliferation. Irradiation likely causes cell metabolism stabilization, which is triggered by light. This is why low doses and short irradiation times are used to provide biostimulation [38]. In the present study, the repeated application of aPDT did not demonstrate significantly decreased BL levels than a single episode of aPDT. These results could justify the administration of a single dose of light for sufficient biostimulation.
Traditional periodontal treatment has some limitations including the decreased effectiveness of mechanical instrumentation in areas like furcation regions that are difficult to access. aPDT is not affected by this limitation because it is based on a photosensitizer agent associated with light emission like laser irradiation. In addition, aPDT presents no side effects, its activity is initiated only with exposure to a light source, and it prevents the selection of resistant bacteria species [46].
Conclusion
In this study, aPDT used in addition to SRP was more effective in the reduction of BL in experimentally induced periodontitis in rats than nonsurgical conventional treatment. However, repeated episodes of aPDT did not improve BL reduction in the furcation region in rats any more than did a single episode of aPDT.
References
Schenkein HA (2006) Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000 2000(40):77–93
Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 2000 36:59–97
Kaldahl WB, Kalkwarf KL, Patil KD (1993) A review of longitudinal studies that compared periodontal therapies. J Periodontol 64:243–253
Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M (1997) Effect of dosimetric and physiological factors on the lethal photosensitization of Porphyromonas gingivalis in vitro. Photochem Photobiol 65:1026–1031
Wilson M (2004) Lethal photosensitization of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem Photobiol Sci 3:412–418
Kömerick N, Nakanishi H, Macrobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47:932–940
Wilson M, Dohson J, Sarkar S (1993) Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 8:182–187
Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q (1998) Photodynamic therapy. J Natl Cancer Inst 90:889–905
Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 4:283–293
Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG (2008) Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontol Res 43:162–167
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG (2007) Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol 78:566–575
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG (2008) In vivo effect of photodynamic therapy on periodontal bone loss in dental furcations. J Periodontol 79:1081–1088
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG (2008) Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol 79:2156–2165
Fernandes LA, de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Martins TM, Okamoto T, Garcia VG (2009) Treatment of experimental periodontal disease by photodynamic therapy in immunosuppressed rats. J Clin Periodontol 36:219–228
Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rossler R, Sculean A (2008) Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized, controlled clinical trial. J Periodontol 79:1638–1644
Theodoro LH, Silva SP, Pires JR, Soares GH, Pontes AE, Zuza EP, Spolidório DM, de Toledo BE, Garcia VG (2011) Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci Jun 18. [Epub ahead of print] doi:10.1007/s10103-011-0942
Sigusch BW, Engelbrecht M, Völpel A, Holletschke A, Pfister W, Schütze J (2010) Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J Periodontol 81:975–981
Andersen R, Loebel N, Hammond D, Wilson M (2007) Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J Clin Dent 18:34–38
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol 35:877–884
Chondros P, Nikolidakis D, Christodoulides N, Rossler R, Gutknecht N, Sculean A (2009) Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: a randomized controlled clinical trial. Lasers Med Sci 24:681–688
Lulic M, Leiggener Görög I, Salvi GE, Ramseier CA, Mattheos N, Lang NP (2009) One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: a proof-of-principle randomized-controlled clinical trial. J Clin Periodontol 36:661–666
Kreisler M, Christoffers AB, Willershausen B, D’hoedt B (2003) Effect of low-level GaAlAs laser irradiation on the proliferation rate of human periodontal ligament fibroblasts: an in vitro study. J Clin Periodontol 30:353–358
Loevschall H, Arenholt-Bindslev D (1994) Effect of low level diode laser irradiation of human oral mucosa fibroblasts in vitro. Lasers Surg Med 14:347–354
Pourzarandian A, Watanabe H, Ruwanpura SM, Aoki A, Ishikawa I (2005) Effect of low-level Er:YAG laser irradiation on cultured human gingival fibroblasts. J Periodontol 76:187–193
Yu W, Naim JO, Lanzafame RJ (1994) The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts. Photochem Photobiol 59:167–170
Pereira AN, Eduardo C de P, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Lubart R, Wollman Y, Friedmann H, Rochkind S, Laulicht I (1992) Effects of visible and near-infrared lasers on cell cultures. J Photochem Photobiol B 12:305–310
Karu TI (1990) Effects of visible radiation on cultured cells. Photochem Photobiol 52:1089–1098
Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T (2002) Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 30:60–66
Sigusch BW, Pfitzner A, Albrecht V, Glockmann E (2005) Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol 76:1100–1105
Shibli JA, Martins MC, Theodoro LH, Lotufo RF, Garcia VG, Marcantonio EJ (2003) Lethal photosensitization in microbiological treatment of ligature-induced periimplantitis: a preliminary study in dogs. J Oral Sci 45:17–23
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28
Okada H, Murakami S (1998) Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med 9:248–266
Biagini G, Checchi L, Miccoli MC, Vasi V, Castaldini C (1988) Root curettage and gingival repair in periodontitis. J Periodontol 59:124–129
Wood S, Nattress B, Kirkham J, Shore R, Brookes S, Griffiths J, Robinson C (1999) An in vitro study of use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J Photochem Photobiol B 50:1–7
Tanaka M, Hanioka T, Takaya K, Shizukuishi S (1998) Association of oxygen tension in human periodontal pockets with gingival inflammation. J Periodontol 69:1127–1130
Garcia VG, de Lima MA, Okamoto T, Milanezi LA, Junior EC, Fernandes LA, de Almeida JM, Theodoro LH (2010) Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: histological study in rats. Lasers Med Sci 25:221–228
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Schaffer M, Bonel H, Sroka R, Schaffer PM, Busch M, Reiser M, Duhmke E (2000) Effects of 780 nm diode laser irradiation on blood microcirculation: preliminary findings on time-dependent T1-weighted contrast-enhanced magnetic resonance imaging (MRI). J Photochem Photobiol B 54:55–60
Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86:694–707
Sibata CH, Colussi VC, Oleinick NL, Kinsella TJ (2000) Photodynamic therapy: a new concept in medical treatment. Braz J Med Biol Res 33:869–880
Raghavendra M, Koregol A, Bhola S (2009) Photodynamic therapy: a targeted therapy in periodontics. Aust Dent J 54(1 Suppl):S102–S109
Kalarová H, Ditrichová D, Wagner J (1999) Penetration of the laser light into the skin in vitro. Lasers Surg Med 24:231–235
Yamammoto Y, Kono T, Kotani H, Kasai S, Mito M (1996) Effect of low-power laser irradiation on procollagen synthesis in human fibroblasts. J Clin Laser Med Surg 14:129–132
Karu T (2003) Low-power laser therapy. In: Karu T (ed) Biomedical Photonics Handbook. CRC Press, Boca Raton, pp 1–24
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22:83–91
Acknowledgments
This study was conducted at the Department of Periodontology, Araçatuba Dental School, State University of São Paulo (UNESP) Araçatuba, São Paulo, Brazil. Mariéllen Longo received a scholarship from the São Paulo State Foundation for Research (FAPESP 2008/10821-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia, V.G., Longo, M., Fernandes, L.A. et al. Treatment of experimental periodontitis in rats using repeated adjunctive antimicrobial photodynamic therapy. Lasers Med Sci 28, 143–150 (2013). https://doi.org/10.1007/s10103-012-1099-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1099-y