Abstract
Diabetes mellitus (DM) is associated with musculoskeletal damage. Investigations have indicated that healing of the surgically tenotomized Achilles tendon was considerably augmented following low-level laser therapy (LLLT) in non-diabetic, healthy animals. The aim of the present study was to evaluate the effect of LLLT on the Achilles tendon healing in streptozotocin-induced diabetic (STZ-D) rats via a biomechanical evaluating method. Thirty-three rats were divided into non-diabetic (n = 18) and diabetic (n = 15) groups. DM was induced in the rats by injections of STZ. The right Achilles tendons of all rats were tenotomized 1 month after STZ injections. The two experimental groups (n = 6 for each group) of non-diabetic rats were irradiated with a helium–neon (He–Ne) laser at 2.9 and 11.5 J/cm2 for ten consecutive days. The two experimental groups of diabetic rats (n = 5 for each group) were irradiated with a He–Ne laser at 2.9 and 4.3 J/cm2 for ten consecutive days. The tendons were submitted to a tensiometric test. Significant improvements in the maximum stress (MS) values (Newton per square millimeter) were found following LLLT at 2.9 J/cm2 in both the non-diabetic (p = 0.031) and diabetic (p = 0.019) experimental groups when compared with their control groups. LLLT at 2.9 J/cm2 to the tenotomized Achilles tendons in the non-diabetic and diabetic rats significantly increased the strength and MS of repairing Achilles tendons in our study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is defined by chronic elevations in blood sugar levels, which ultimately cause serious long-term complications throughout the body [1]. DM is a relatively common disease with major public health implications. Although much has been written regarding the clinical manifestations, treatment, and pathology of DM, it remains a major health concern due to its rising rates around the world [2]. Boyle et al. have reported that the annual diagnosed DM incidence will increase from about eight cases per 1,000 in 2010 to about 15 per 1,000 in 2050 [3]. Not only is DM associated with musculoskeletal [4, 5], microvascular, and macrovascular damage (which accounts for DM-related morbidity and mortality) but it also causes such complications as retinopathy, arteriopathy, peripheral neuropathy, and poor wound healing [6]. Diabetics are also more prone to develop problems with musculoskeletal systems than normal people [7–10].
Tendons are soft connective tissues that consist of parallel collagen fibers embedded within an extracellular matrix. This organized structure allows tendons to withstand and transmit large forces between the muscle and bone [11]. DM causes a wide range of musculoskeletal disorders, including calcific shoulder periarthritis (tendinitis) [12], Achilles tendon thickening [13], wound complications after tendon repair [14, 15], and tendon ruptures [16, 17].
Some studies have indicated that streptozotocin-induced diabetic (STZ-D) rats serve as useful models for the mechanisms related to tendon healing [18–20]. The clinical application of low-level laser therapy (LLLT) is growing rapidly [21]. In 1968, Mester and colleagues have first reported the earliest clinical application of LLLT [22].
The initial works of Enwemeka’s laboratory indicated that healing of surgically tenotomized Achilles tendons was considerably augmented following helium–neon (He–Ne) laser treatments [23–25]. In these studies, the beneficial effects of LLLT were studied by analyzing morphometrical, ultrastructural, and biomechanical evaluations in healing Achilles tendons in rabbits [23–25].
Since non-diabetic wounds generally heal well without any intervention, healing impaired wounds provides a better model to evaluate the potential beneficial effects of LLLT [26]. It is well-known that diabetes interferes with the normal tendon and tendon healing process [12–20, 27] and thus poses a serious challenge in clinical medicine. A review of the available literature has shown that although the effects of LLLT on tendon injuries have been thoroughly studied in non-diabetic animals [23–25, 28–30], the impact of LLLT on the Achilles tendon healing in STZ-D rats has not yet been characterized. Only some studies have reported positive effects of LLLT on diabetic skin wound healing in rats [26, 31]. Such information will assist clinicians with their choice of treatment for tendon healing in diabetic patients. The present study determined the effects of LLLT with a He–Ne laser on the healing of completely transected Achilles tendons in STZ-D rats.
Materials and methods
Animals and study design
A total of 33 5-month-old male Wistar rats weighing about 300 g were obtained from the Pasteur Institute of Iran, Tehran, Iran. Rats were randomly divided into group 1 (non-diabetic, LLLT with 2.9 J/cm2), group 2 (non-diabetic, LLLT with 11.5 J/cm2), group 3 (non-diabetic, no LLLT), group 4 (diabetic, LLLT with 2.9 J/cm2), group 5 (diabetic, LLLT with 4.3 J/cm2), and group 6 (diabetic, no LLLT). Rats were housed individually in standard rat cages made of polypropylene in a 12-h light/dark environment. The animals were provided with water and standard food ad libitum. All procedures were approved by the Medical Ethics Committee of Shaheed Beheshti University MC (protocol no. 13/15183) Tehran, Iran. There were six rats in each of the non-diabetic groups and five in each of the diabetic groups.
Induction of type I diabetes
Type I diabetes was induced in groups 4, 5, and 6 by intraperitoneal injections of the pancreatic β-cell toxin STZ (Zanosar Pharmacia and Upjohn Co, Kalamazoo, MI, USA) freshly dissolved in sterile distilled water (pH = 7.3) at a single dose of 55 mg/kg body weight [32]. The non-diabetic rats received control injections of distilled water. Diabetes was defined as a blood glucose concentration greater than 250 mg/dl in a distal tail small injury sample (GM 300, Biomince, GM H, Heerbrugg, Switzerland) 7 days after STZ injection [32]. The blood glucose level and body weight of the rats were monitored every 2 weeks throughout the study. All diabetic rats were kept for 30 days after the STZ injection before being sacrificed on day 40 after the STZ injection for tensiometric examination [20]. Four diabetic rats died during the experiment due to unknown reasons and were replaced. Diabetes was induced in all the new rats by using the same procedures.
Tenotomy
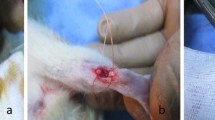
Rats were anesthetized by using 50 mg/kg ketamine hydrochloride intramuscularly injected along with 5 mg/kg diazepam. The skin cover around the right Achilles tendon was shaved and thoroughly scrubbed. The tendon was approached by a medial skin incision and then released from the surrounding soft connective tissue via this incision. The tendinous portion of the plantaris was removed to prevent any possibility of an internal splint. The Achilles tendon was cut sharply and transversely with a scalpel 5 mm above its insertion into the calcaneus. Both ends of the transected Achilles tendon were approximated and immediately repaired with 4.0 nylon, using a modified Kessler suture technique, and the skin was then sutured. No operation was performed on the left uninjured hind limb, no cast or dressing was applied, and the animals were unrestricted during the healing phase [20, 33, 34].
LLLT
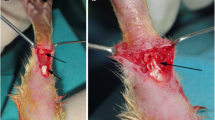
We used a He–Ne laser (THORLAB, USA) with 632.8 nm wavelength, 7.2 mW average power, circular beam shape, 0.00524 cm2 surface area, and 1.4 W/cm2. LLLT was started immediately after surgery and skin suturing (day 0). Energy densities of 2.9 J/cm2 were used for 2 s to irradiate each point in groups 1 and 4, 11.5 J/cm2 was used for 8 s to irradiate each point in group 2, and 4.3 J/cm2 for 3 s was used to irradiate each point in group 5. The surface area of the target tissue was comprised of the repairing tissue at the center and both ends of the transected tendon in its proximal and distal positions (Fig. 1a); sequential treatment was undertaken to ensure that every unit area received a similar dosage of light energy [32, 35]. The target tissue area was considered to be an 11 × 4-mm rectangle, divided equally into 44 points. The laser probe was positioned perpendicularly near (<1 cm) the surface of the target tissue (repairing Achilles tendon) [32, 35]. Sequential treatment was commenced from point no. 1 and terminated at point no. 44 (Fig. 1a). During treatment, the irradiated animals were sedated by 1/2 dose of anesthetizing drugs. Animals were kept in a special restrainer, from which their forelimbs and hind limbs were extended by extension at the knee joints and plantar flexion in the ankle joints.
a Schematic depiction of the repairing Achilles tendon and laser-treated points. Laser irradiation was commenced at point no. 1; continued at point nos. 4 and 5, 41; and terminated at point no. 44. b Schematic representation of the load–deformation curve of the repairing Achilles tendon. The graph illustrates ML derived from the material testing machine and its computer
Biomechanical test
On day 10 [36], the rats were killed by chloroform inhalation in a closed space. Each tendon was excised from the musculotendinous junction proximally after it had been carefully dissected from the surrounding tissues. The tendon with the attaching calcaneus (bone) was removed [36]. The excised tendons were frozen in 0.9% NaCl solution at −20°C. On the day of the biomechanical test, specimens were retrieved from the freezer and allowed to thaw at room temperature. After thawing, specimens were kept moistened with a 0.9% NaCl solution. The transverse and anterior–posterior diameters of the specimens (repairing tissue) were measured as an index of the cross-sectional area (thickness), by a digital caliper. The musculotendinous end was fixed between two paper strips to the upper jaw, and the calcaneus was mounted onto the lower jaw of the machine. Biomechanical measurements were carried out using a material testing machine (Zwick/Roell, Germany). Tensile load was then applied at a displacement rate of 1 mm/s, and the load characteristics were directly plotted on an X–Y chart recorder. A typical load–deformation curve, identifying both elastic (linear) and plastic resistance components until failure, separated by “yielding point” (departure from linearity), is depicted in Fig. 1b.
From the load–deformation curve, the following biomechanical properties were automatically calculated:
-
1-
Maximum load (ML; Newton) was measured directly from the load–deformation curve; it represented the maximum tensile force applied to rupture the specimen.
-
2-
Maximum stress (MS; Newton per square millimeter) was calculated as the maximum load divided by the cross-sectional area of the tendon [20, 26, 31, 34].
Statistical analysis
Data were analyzed by both the paired and independent sample Student t tests. In all analyses, p < 0.05 was considered statistically significant. The data of groups 1, 3, 4, and 6 were subjected to one-way of analysis variance (ANOVA) too. Multiple comparisons were performed using the least significant difference (LSD). The data were expressed as mean ± SD.
Results
General observations
There was no evidence of infection in the tenotomized rats during laser treatments. All 15 rats in groups 4, 5, and 6 developed clinical evidence of diabetes following STZ injections. Our paired Student t test showed that the body weights of the diabetic rats significantly decreased at the time of tendon sampling when compared to the beginning of the study (322.7 ± 13.7 vs. 225.8 ± 20.1; p = 0.002). Diabetic rats were noted to have frequent urination during the course of study, which was a sign of uncontrolled diabetes. Blood glucose levels rose to 301.6 ± 35.9 mg/dl in the diabetic rats 1 week after STZ injections and further increased to 460.8 ± 66.6 mg/dl at the time of tendon sampling.
Biomechanical test
Biomechanical analyses of the repairing Achilles tendons in the diabetic rats showed that LLLT significantly increased both ML and MS in group 4 when compared with the non-irradiated control diabetic rats from group 6. This examination yielded an ML of 25.1 ± 4.9 N for the diabetic laser-treated tendons and an ML of 9.4 ± 6.6 N for diabetic control tendons (p = 0.003). MS was 2.7 ± 0.5 N/mm2 for the diabetic laser-treated tendons and 1.8 ± 0.9 N/mm2 for the diabetic control tendons (p = 0.019; Figs. 2 and 3).
Biomechanical analysis of the repairing Achilles tendons indicated that LLLT significantly increased MS of the laser-treated rats in group 1 when compared with the non-irradiated control rats in group 3 (5.0 ± 0.98 vs. 2.6 ± 1.6 N/mm2; p = 0.031; Fig. 3). Statistical analysis of the repairing Achilles tendons revealed that LLLT did not increase any of the biomechanical parameters of the laser-treated non-diabetic rats from group 2 compared with the non-diabetic control rats of group 3. LLLT significantly increased ML of the repairing Achilles tendons in the laser-treated diabetic rats of group 5 compared to the non-irradiated diabetic rats of group 6 (p = 0.015; Fig. 2).
ANOVA showed LLLT significantly increased ML of group 1 than that of group 6 (LSD test: p = 0.005). ML of group 4 was significantly higher than those of groups 3 and 6 (LSD test: p = 0.028 and p = 0.002, respectively). ANOVA showed LLLT significantly increased MS of group 1 than those of groups 3, 4, and 6 (LSD test: p = 0.004, p = 0.006, p = 0.000, respectively).
Discussion
Our results show that LLLT, at the wavelength and energy densities used in the present work, improved the healing process of tenotomized Achilles tendons as evaluated by a biomechanical test of the repairing tendon in non-diabetic and diabetic rats. The best significant response was observed in the rats of groups 1 and 4. The biomechanical test has already been utilized in the evaluation of tendon injury in studies on laser-treated animals [24, 29, 37].
In our non-diabetic rats, the repairing Achilles tendons showed a significant increase in MS with LLLT at the 2.9-J/cm2 energy density on day 10 post-injury when compared with its relevant control. Our biomechanical analysis indicated that the repairing Achilles tendons in the non-diabetic rats who received laser treatments were stronger when the ML (strength) was normalized for variation in size of the injury site of the tendon (MS). This finding proved that an optimal energy density of LLLT was effective in tendon healing and significantly increased MS of the repairing Achilles tendon in non-diabetic rats, which supported the findings of previous studies [24, 29, 37].
The effects of 1-, 2-, 3-, 4-, and 5-mJ/cm2 He–Ne and Ga–As lasers [24] as well as the 1-J/cm2 He–Ne laser [29] on surgically tenotomized Achilles tendons in rabbits have been previously studied. In these studies, the operated hind limbs were immobilized. Enwemeka [24] showed that, regardless of the type of laser used, exposure to the laser beam enhanced the tensile stress but not the ultimate tensile strength and energy absorption capacity of tendons. Elwakil found better biomechanical properties of laser-treated tendons with statistical significance (p ≤ 0.01) for most of the biomechanical parameters [29].
In our non-diabetic rats, the results from the biomechanical test indicated that the repairing Achilles tendons showed a significant increase in MS with LLLT at the 2.9-J/cm2 energy density at day 10 post-injury when compared with the relevant control. In addition, a marginal increase in ML was observed. In our diabetic rats, the results from the biomechanical test revealed that the He–Ne laser at 2.9 J/cm2 (group 4) and 4.3 J/cm2 (group 5) energy densities significantly accelerated tendon healing. The best response was observed in the laser-treated animals from group 4. The laser-treated animals in group 5 exhibited a significant increase in ML compared to the controls. The laser-treated animals in group 4 showed a significant increase in ML and MS compared to the controls.
A reason for the lack of statistically significant improvement in all biomechanical parameters with LLLT in the non-diabetic rats of the current study and the statistically significant increase in most biomechanical parameters of the diabetic rats of this study may be due to evidence that the effect of LLLT is also dependent on the physiologic state of the tissue at the exposure time [38, 39]. In the current study, although treatment at 2.9 J/cm2 only significantly increased MS in the non-diabetic rats, it seems to have caused a significant increase in ML and MS of the in the diabetic rats.
There is a wide variation in recommendations for the optimal energy density of LLLT (1 mJ/cm2 to 10 J/cm2) for a trans-sectional model of the Achilles tendon injury [23, 30]. Generally, a 632.8–904-nm wavelength laser is used [28, 40]. For the He–Ne laser, a 632.8-nm wavelength is most frequently employed for Achilles tendon healing [23–25, 28, 29]. Thus, we used a laser with a wavelength of 632.8 nm and energy densities of 2.9–11.5 J/cm2.
The results of the present study on non-diabetic rats agreed with those of other studies on the benefits of the use of LLLT for the treatment of the tenotomized Achilles tendon injury [23–25, 28–30, 40]. A salient finding of our work was that the laser-treated rats at 2.9 J/cm2 (diabetic rats, group 4) had a significantly higher ML (p = 0.003) and MS (p = 0.019) when compared with the control group (Figs. 2 and 3). The remaining laser-treated groups (diabetic rats of group 5 and non-diabetic rats of groups 1 and 2) had higher biomechanical values than their controls, but it was not statistically significant, with the exception of MS in group 1 (p = 0.031) and ML in group 5 (p = 0.015). This suggests that further studies with a larger number of animals are necessary to demonstrate whether LLLT can significantly improve the biomechanical parameters of the repairing Achilles tendon in diabetic rats.
Our results are in agreement with those of other studies on the benefits of the use of LLLT for the treatment of skin wounds in diabetic animals [26, 31]. Healing an impaired system, such as seen with STZ-D animals, provides a situation in which the effect of LLLT may be pronounced [26, 31]. In STZ-D, LLLT has been shown to significantly improve the tenotomized Achilles tendon at day 10 post-injury. Day 10 is a critical point during the tendon healing process, when the tendon has achieved significant strength (failure load 50–61% of the intact tendon) but is susceptible to retear. Similarly, the results of the Yuan et al. study have demonstrated that NO-flurbiprofen significantly enhanced the biomaterial properties of the healing tendon (maximum load/cross-sectional area) on postoperative day 10 [36].
The precise mechanism behind the improvement in tendon healing observed in laser-treated non-diabetic and laser-treated diabetic rats is unknown. LLLT has been shown to facilitate collagen production [23–25, 28, 29] and aggregation of collagen bundles [30], increase the biomechanical parameters of the transected Achilles tendon [24, 29], and reduce mRNA expression for pro-inflammatory mediators [41] in non-diabetic animals.
Conclusion
In light of the findings of the present study, we conclude that LLLT with the He–Ne laser significantly increased the biomechanical parameters of the repairing Achilles tendon in both non-diabetic and diabetic rats 10 days after complete tendon transection. The LLLT effects were dependent on the energy density of the laser. A lower dose (2.9 J/cm2) has produced a more desirable outcome, particularly in our diabetic rats. More researches with keeping rats for 4 and 8 weeks after surgery and laser irradiation with higher doses, more than 40 J/cm2, are suggested
References
Kemmis K (2010) Common musculoskeletal disorders in older adults with diabetes. Top Geriatr Rehabil 26:264–272
Perkins I (2004) Diabetes mellitus epidemiology—classification, determinants and public health impacts. J Miss State Med Assoc 45:355–362
Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF (2010) Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 8:29–41
Ramchurn N, Mashamba C, Leitch E, Arutchelvam V, Narayanan K, Weaver J, Hamilton J, Heycock C, Saravanan V, Kelly C (2009) Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med 20:718–721
Cagliero E, Apruzzese W, Perlimutter G, Nathan DM (2002) Musculoskeletal disorders of the hand and shoulder in patients with diabetes mellitus. Am J Med 20:718–721
Chaturvedi N (2007) The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract 76(suppl 1):S3–S12
Marks RM (2001) Complications of foot and ankle surgery in patients with diabetes. Clin Orthop Relat Res 391:153–161
Ganesh SP, Pietrobon R, Cecilio WA, Pan D, Lightdale N, Nunley JA (2005) The impact of diabetes on patient outcomes after ankle fracture. J Bone Joint Surg Am 87:1712–1718
Akturk M, Karaahmetoglu S, Kacar M, Muftuoglu O (2002) Thickness of the supraspinatous and biceps tendons in diabetic patients. Diabet Care 25:408
Smith LL, Burnet SP, Mc Neil JD (2003) Musculoskeletal manifestations of diabetes mellitus. Br J Sports Med 37:30–35
Lin TW, Gardenas L, Soslowsky LJ (2004) Biomechanics of tendon injury and repair. J Biochem 37:865–877
Mavrikakis ME, Drimis S, Kontoyannis DA, Rasidakis A, Moulolpoulou ES, Kontoyannis S (1989) Calcific shoulder periarthritis (tendinitis) in adult onset diabetes mellitus: a controlled study. Ann Rheum Dis 48:211–214
Akturk M, Ozdemir A, Maral I, Yektin I, Arslan M (2007) Evaluation of Achilles tendon thickening in type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 115:92–96
Chen AL, Shapiro JA, Ahn AK, Zuckerman JD, Cuomo F (2003) Rotator cuff repair in patients with type 1 diabetes mellitus. J Shoulder Elbow Surg 12:416–421
Bruggeman NB, Turner NS, Dahm DL, Voll AE, Hoskin TL, Jacofsky DJ, Haidukewych GJ (2004) Wound complications after open Achilles tendon repair: an analysis of risk factors. Clin Orthop Relat Res 427:63–66
Didomenico LA, Williams K, Petrolla AF (2008) Spontaneous rupture of the anterior tibial tendon in a diabetic patient: results of operative treatment. J Food Ankle Surg 47:463–467
Maffulli N, Longo UG, Maffulli GD, Khanna A, Denaro V (2011) Achilles tendon ruptures in diabetic patients. Arch Orthop Trauma Surg 131:33–38
Chbinou N, Frenette J (2004) Insulin dependent diabetes impairs the inflammatory response and delays angiogenesis following Achilles tendon injury. Am J Physiol Regul Integr Comp Physiol 286:R952–R957
Bedi A, Fox AJ, Harris PE, Deng XH, Ying L, Warren RF, Rodeo SA (2010) Diabetes mellitus impairs tendon-bone healing after rotator cuff repair. J Shoulder Elbow Surg 19:978–988
Bayat M, Feridoni MJ, Piryaie A, Dadpay M, Gazour R, Rezaei F, Norouzian M, and Akbari M (2012) Effect of streptozotocin induced type-1 diabetes on tendon healing in rats, a biomechanical and histological study. Int J Diabetes Mellit (in press)
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22:141–150
Mester E, Juhasz J, Veraga P, Karika G (1968) Laser in clinical practice. Acta Chir Acad Sci Hung 9:349–357
Enwemeka CS, Rodriguez O, Gall NG, Walsh NE (1990) Morphometric of collagen fibril population in He–Ne laser photostimulated tendons. J Clin Laser Med Surg 8:47–51
Enwemeka CS (1991) Connective tissue plasticity: ultrastructural, biomechanical, and morphometric effects of physical factors on intact and regenerating tendons. J Orthop Sports Phys Ther 14:198–212
Enwemeka CS (1992) Ultrastructural morphometry of membrane-bound intracytoplasmic collagen fibrils in tendon fibroblasts exposed to He–Ne laser beam. Tissue Cell 24:511–523
Reddy GK (2003) Comparison of the photostimulatory effects of visible He–Ne and infrared Ga–As lasers on healing impaired diabetic rat wounds. Lasers Surg Med 33:344–351
Fox AJ, Bedi A, Deng SH, Ying L, Harris PE, Warren RF, Rodeo SA (2011) Diabetes mellitus alters the mechanical properties of the native tendon in an experimental rat model. J Orthop Res 29:880–885
Reddy GK, Stehno-Bittel L, Enwemeka CS (1998) Laser photostimulation of collagen production in healing rabbit Achilles tendon. Lasers Surg Med 22:281–287
Elwakil TF (2007) An in vivo experimental evaluation of He–Ne laser photostimulation in healing Achilles tendons. Laser Med Sci 23:53–59
Carrinho PM, Renno AC, Koeke P, Salate ACB, Parizotto NA, Vidal BC (2006) Comparative study using 685-nm and 830-nm lasers in tissue repair of tenotomized tendons in the mouse. Photomed Laser Surg 24:754–758
Reddy GK, Stenho-Bittel L, Enwemeka CS (2001) Laser photostimulation accelerates wound healing in diabetic rats. Wound Rep Reg 9:248–255
Bayat M, Abdi S, Javadieh F, Mohsenifar ZH, Rashid MR (2009) The effects of low-level laser therapy on bone in diabetic and non diabetic rats. Photomed Laser Surg 27:703–708
Lin JH, Wang MX, Wei A, Zhu W, Diwan AD, Murrell GA (2001) Temporal expression of nitric oxide synthesis isoforms in healing Achilles tendon. J Orthop Res 19:136–142
Zhang F, Liu H, Stile F, Lei MP, Pang Y, Oswaldt TM, Beck J, Dorsett-Martin W, Lineaweaver WC (2003) Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg 112:1613–1619
Enwemeka CS (2009) Intricacies of dose in laser phototherapy for tissue repair and pain relief. Photomed Laser Surg 27:387–393
Yuan J, Murrel GA, Wei AQ, Appleyard RC, DelSoldato P, Wang MX (2003) Addition of nitric oxide via nitroflurbiprofen enhances the material properties of early healing of young rat Achilles tendons. Inflamm Res 52:230–237
Demir H, Menku P, Kirnap M, Calis M, Ikizceli I (2004) Comparison of the effects of laser, ultrasound and combined laser + ultrasound treatments in experimental tendon healing. Lasers Surg Med 35:84–89
Karu TI (1990) Effect of visible radiation on cultured cells. Photochem Photobiol 52:1089–1098
Steinlechner C, Dyson M (1993) The effect of low-level laser therapy on the proliferation of keratinocytes. Laser Ther 5:65–73
Fillipin LI, Mauriz JL, Vedovelli K, Moreira AJ, Zettler CG, Lech O, Marroni NP, Gonzalez-Gallego J (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Pires D, Xavier M, Araujo T, Silva JA Jr, Aimbire F, Albertini R (2011) Low-level laser therapy (LLLT, 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94
Acknowledgments
We wish to extend our sincere thanks to the late Mrs. Jamileh Rezaei and the Vice-Chancellor of Research at the Medical Faculty of Shaheed Beheshti University MC, Tehran, Iran for financial support (grant no. 13.15183).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nouruzian, M., Alidoust, M., Bayat, M. et al. Effect of low-level laser therapy on healing of tenotomized Achilles tendon in streptozotocin-induced diabetic rats. Lasers Med Sci 28, 399–405 (2013). https://doi.org/10.1007/s10103-012-1074-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1074-7