Abstract
Laser light can be used during endodontic procedures to sterilize the root canal by destroying bacteria. Previous in-vitro studies that investigated the mechanism of the destruction of bacteria inhabiting the root canal by 1,064-nm Nd:YAG and 808-nm diode laser light used substrates that absorb light in the near-infrared (NIR) spectrum. These substrates heat the bacterial microenvironment, which possibly contributes to cell death. To determine the direct effect of laser light on the bacterial sample in the absence of detrimental heating, a sapphire substrate, which is virtually transparent in NIR spectrum, was inoculated with bacterial samples and subjected to laser irradiation at 1,064 nm (1.5 W, 15 Hz) and at 808 nm (1.5 W, 20 Hz). Enterococcus faecalis, Escherichia coli, and Porphyromonas gingivalis bacteria were used. E. faecalis and E. coli were largely unaffected by laser light. The viability of P. gingivalis, a pigmented bacterium, was directly affected by both NIR wavelengths (a 57% decrease of viability at 1,064 nm and a 31% decrease at 808 nm). Our results indicate that the primary mediator of cell death appears to be the interaction between NIR laser light and the bacterial microenvironment, most likely in the form of heating. Our research suggests that when optimizing the efficacy of laser-assisted endodontic sterilization of the root canal, the optical characteristics of the bacterial microenvironment play a key role, as nonpigmented bacteria appear to be virtually transparent at 808 nm and 1,064 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After infection of the pulpal tissue, bacteria also begin to penetrate into the deeper layers of root dentin and so propagate a periapical inflammation with subsequent destruction of the adjacent connective tissues [1]. Rinsing solutions, such as NaOCl, which are applied during conventional root canal treatment, act through direct contact with the targeted bacteria and are unable to affect microorganisms in the deeper layers of dentin due to the insufficient penetration depth of the bactericidal solutions [2, 3]. In addition, bacteria like E. faecalis are highly resistant to various chemical disinfectants used in endodontics today and present an additional challenge [4]. E. faecalis is known for its ability to form intra- and extra-radicular biofilms, which makes it extremely difficult to control [5–8] in addition to its various resistances toward chemicals disinfectants, high temperature, and desiccation [4, 9–11]. E. faecalis is the predominant bacterial species found in persistent endodontic infections [12].
The introduction of lasers in the field of endodontics brought about an increase in the effectiveness and success rate of root canal treatment, due to the laser's ability to affect deeper parts of dentinal tissues [13–15]. Nd:YAG [16–18] and diode lasers [19–22] were among the first lasers to be scientifically evaluated in clinical studies and both have gained widespread acceptance in the field of laser-assisted endodontics due to their disinfecting capabilities [23–25].
The mechanism of NIR laser-mediated destruction of bacteria has not been determined. Two possible mechanisms can be considered. In the first mechanism, the laser light is strongly absorbed in the substrate onto which the bacteria adhere (in this case dentin). The resultant heating of the substrate causes a local rise in temperature high enough to result in the death of the attached micro-organisms. The second possible mechanism suggests that the laser light is absorbed by the bacteria and is therefore responsible for direct damage to the bacterial cell. With regard to the former, the temperature rise recorded in a previous study [23] cannot explain the bactericidal effect. With regard to the latter, no studies, to the best of our knowledge, have been conducted to test the direct effect of NIR lasers, with the same or very similar laser parameters to the ones being used in clinical practice, on bacteria, especially E. faecalis, with emphasis on excluding the potential bactericidal effect of the substrate, on which the bacteria are attached (i.e., the heating of substrate via laser light). To address this issue, an in-vitro study was performed to evaluate the potential direct bactericidal effect of two NIR lasers on bacteria, a 1,064-nm Nd:YAG laser and a 808-nm diode laser under standardized conditions. In particular, attention was paid to the choice of substrate onto which the bacteria would be placed. The substrate had to posses a high transmission rating in NIR spectra in order to absorb as little laser light as possible and thus minimizing its heat build-up. Viability of bacterial samples was tested with the flow cytometry method in addition to the standard plate count to compensate for some of the shortcomings of the plate count method.
Material and methods
Sapphire windows
Sapphire windows of appropriate dimensions for the experiment were chosen as a substrate because of their high transmission for NIR wavelengths (i.e., the windows do not heat up or the heating is negligible when irradiated by the laser parameters used in this study). The uncoated circular sapphire windows (Techspec® sapphire windows, 7.5 mm diameter, 0.35 mm thickness; Edmund Optics) were inspected and cleaned if necessary. They were then sterilized by autoclaving to destroy any bacteria on their surface. Sterility was verified by plate culture and flow cytometry methods.
Bacterial inoculation
Sterilized sapphire windows were first coated with 2 μl of inactivated FBS (fetal bovine serum), which was allowed to dry for approximately 15 min in a laminar flow cabinet. The dried inactivated FBS prevents the bacteria from adhering too strongly to the sapphire surface. This substantially increases the portion of bacteria that can be harvested for further analysis with methods such as vortexing. Following coating, the windows were inoculated with 2 μl of bacterial test strains suspensions: E. coli (ATCC 25922), E. faecalis (ATCC 29212), or P. gingivalis (ATCC 33277) on one side by means of a micropipette (each test strain on its own sapphire window). Concentrations used were 1–3 × 108 CFU/ml for the E. coli and E. faecalis and 2 × 106 CFU/ml for P. gingivalis. E. coli was used as a model Gram-negative bacteria for the case that there would be any differences in absorption due to different cell-wall composition. E. faecalis was used as a model Gram-positive bacteria and P. gingivalis was used as a model pigmented bacteria (black pigment), as both E. faecalis and E. coli are unpigmented. The droplet containing the bacterial suspension was dried under laminar flow for approximately 10 min in a laminar flow cabinet to evaporate the excess water.
Laser systems
An AT Fidelis laser (Fotona d.d., Ljubljana) was used as the Nd:YAG laser source (1,064 nm). A Fotona XD-2 laser (Fotona d.d., Ljubljana) served as the diode laser source (808 nm). The laser could be operated in pulsed or continuous wave (CW) mode.
Each laser was equipped with a proprietary flexible waveguide (laser fiber with a 300-μm fiber tip diameter) and was operated in pulsed mode (15 Hz for the Nd:YAG, 20 Hz for the diode laser) without any water spray or air cooling. The lasers were adjusted for an effective average output power of 1.5 W measured directly on the fiber tip using a wattmeter (Coherent, Inc., Santa Clara, CA, USA) before each irradiation cycle. This procedure ensured stable and standardized irradiation schemes for each sample.
Laser irradiation
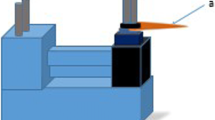
After incubation, 20 samples of each of the bacterial strains underwent laser irradiation (ten of the samples with the Nd:YAG laser and ten with the diode laser). Ten samples served as the control group for each test strain and remained untreated. The following irradiation procedure was used with both laser sources: The specimens were irradiated from the side opposing the inoculated area in near-contact mode. The fiber was mounted in a specially designed mount that enabled constant circular scanning movement of the optical fiber 0.7 mm above the window surface to cover the entire inoculated area. A distance of 0.7 mm was chosen to avoid potentially burning the fiber tip onto the surface of the sapphire window. The fiber tip was held perpendicular to the irradiated area, thus maximizing the exposure of the bacteria to the laser light (Fig. 1). One lasing cycle comprised five irradiations of 5 s each, with 15-s intervals in between. No water or air cooling was used with any of the devices. This irradiation protocol is very similar to the procedure described in [23] and to the actual irradiation procedure used in clinical practice. The sapphire windows were placed in specially designed sterilized holders, which attach to the outside rim of the sapphire window.
Bacteriological evaluation
Upon irradiation, the specimens were placed into sterile 5-ml Falcon tubes (BD Biosciensces) and 1 ml of phosphate buffered saline (PBS) solution was added. Each tube was then vortexed for 1 min to wash the bacteria from the sapphire windows.
Both standard culture plate and flow cytometry methods were used in parallel to determine the number of viable bacteria.
Next, 500 μl of PBS solution was diluted in log 10 steps. Then, 10 μl of each dilution were applied to culture plates (E. faecalis, E. coli – blood agar; P. gingivalis – Schaedler agar) and incubated (E. coli, E. faecalis – 24 hours; P. gingivalis – 7 days, anaerobically) at 36.5°C. The colonies were then counted and the total number of bacteria (colony forming units per milliliter) was assessed.
A total of 500 μl of the PBS solution was used for flow cytometry analysis. The Cell Viability Kit with Liquid Counting Beads (BD Biosciences) was used according to the manufacturer's instructions to determine the number and viability of the bacteria. The kit contains thiazole orange (TO) solution to stain all cells and propidium iodide (PI) to stain dead cells as well as a liquid suspension of fluorescent beads, which are added to the flow sample to calculate absolute counts. Live cells have intact membranes and are impermeable to dyes such as PI, which leaks into cells with compromised membranes. TO is a permeant dye and enters all cells, live and dead, to varying degrees. The fluorescent signal from TO in viable cells allows their enumeration even when debris in the cell preparation contaminates a scatter gate around the cells. Thus the combination of these two dyes provides a rapid and reliable method for discriminating live and dead cells [26]. The advantage of the flow cytometry method over the culture plate method is mainly in the speed of the procedure; results are available within minutes as opposed to 24-h or longer incubation times when working with culture plates.
Statistical analysis
We used SPSS software to perform the statistical analysis. After appropriate assumptions were checked for all the variables tested, we used Student's t test to compare pairs of data measured by plate count and flow cytometry methods irradiated with the same laser type to compare both methods for viability determination (e.g., flow cytometry and plate count) as well as pairs of data where viability was measured by the same method to see if there was any statistically significant difference between the two lasers used. Statistical significance was set at p < 0.05.
Results
Figures 2, 3, and 4 show the results of the bacteriologic test regarding E. coli, E. faecalis, and P. gingivalis. Samples are rated in percentage of viable bacteria/colony counts (flow cytometry/plate count technique), and the laser device used.
For E. faecalis, the Nd:YAG laser induced a <1%/7% reduction (flow cytometry/plate count) and the diode laser a 5%/5% reduction. With regard to E. coli, the Nd:YAG laser caused a 25%/15% reduction and the diode laser a 19%/14% reduction. For the pigmented P. gingivalis, the Nd:YAG laser caused a 57% reduction and the diode laser a 31% reduction as measured by flow cytometry. No growth of P. gingivalis was observed on Schaedler agar plates in all of the irradiated samples. All viability percentages are an average of ten irradiated samples (per bacterial strain) compared to an average of ten control samples (per bacterial strain).
Discussion
Bacterial colonization of the root canal and its associated tubular network can present a serious challenge to successful endodontic treatment. Bacterial species such as E. faecalis can prove especially problematic to eliminate, resulting in persistent endodontic infections [8, 12]. These bacteria have numerous resistances, biofilm formation capability and have adapted to conditions in root canal and adjacent spaces. Conventional root canal treatment is centered around the removal of the infected pulp and dentin layers by using mechanical techniques and bactericidal irrigants. These conventional techniques have limitations. According to Kouchi et al. [3], bacteria are capable of invading the periluminal dentin up to a depth of 1.1 mm. Chemical disinfectants penetrate no more than 0.13 mm into the dentin as described by Berutti et al. [2]. This implies that a dentin layer of almost 1-mm thickness can serve as a reservoir for bacteria, possibly resulting in long-term treatment failures.
To remedy this situation, various laser systems were evaluated for use in endodontics. The first wavelength researched was 1,064 nm Nd:YAG; bactericidal effects were demonstrated [23, 24]. The diode laser was at first limited to soft-tissue applications, but was later also proven to be effective in endodontic procedures [19–22]. Both wavelengths showed effectiveness, even through 1-mm thickness of dentin [23].
As seen from the results in our study, P. gingivalis was directly affected by both wavelengths, resulting in an average decrease of 57% in viability when using Nd:YAG laser and a 31% decrease when a 810-nm diode laser was used. The most likely reason for this is the presence of black pigment (protoporphyrin IX) in P. gingivalis, which can absorb a portion of NIR wavelength energy.
E. coli and E. faecalis, on the other hand, appear to be almost completely transparent to the 810-nm and 1,064-nm wavelengths as both wavelengths had negligible direct bactericidal effects. The bactericidal effect was slightly more pronounced in the E. coli group, but the most likely explanation for both the E. faecalis and E. coli viability decrease is the effect of desiccation on bacterial cells during the experiment. The rate of evaporation of excess water from the 2 μl of bacterial suspension varied slightly from sample to sample. Gram-positive bacteria such as E. faecalis are more resistant to desiccation due to the structure of their cell wall when compared to Gram-negative bacteria such as E. coli. But even if we could attribute the bactericidal effect on E. coli and E. faecalis solely to laser action, it is still relatively negligible; under 10% for E. faecalis.
The results obtained from flow cytometry and plate count are in very good agreement for E. faecalis and E. coli. The determination and counting of viable/dead bacteria with the use of flow cytometry has the advantage of being faster since 24-h or longer incubation periods are not needed. It is also possible to see the total number of bacteria and their composition (i.e., the ratio of viable/dead cells), which were successfully removed from the surface to which they were attached. The results between the two cell counting methods are not in good agreement for P. gingivalis, where irradiated samples showed no growth on Schaedler agar plates. One possible explanation is that there was laser-induced damage to the cells, which resulted in inhibited growth on agar plates.
According to results obtained in this study, we can conclude that the direct bactericidal effect of Nd:YAG and 810-nm diode lasers on the tested nonpigmented bacteria is unlikely. A potentially bactericidal mechanism of laser action in the IR spectra has been observed in optical traps and was described as an undefined type of photodamage. The mechanism remains poorly understood although it is apparent that the presence of oxygen in the bacterial microenvironment is required [27, 28]. Also, the laser parameters used in optical traps differ from those routinely used in endodontic practice. Another option to achieve a bactericidal effect in the deeper layers of dentin with the Nd:YAG and the 810-nm diode laser is to heat up the substrate, to which the bacteria are attached or to introduce a photosensitizer into bacterial cells. The introduction of a photosensitizer to the inside of dentinal tubules and ensuring its selective absorption onto/in bacterial cells would be a very daunting task. In order to eliminate bacteria, such as E. faecalis via heating of its surroundings, a relatively high temperature would be required, if we consider that the aforementioned bacteria can tolerate 60°C for 30 min [29]. We thus need to consider that temperature spikes in irradiated parts of the dentin only last for a fraction of a second due to the nature of the way the laser energy is delivered. To avoid excessive bulk heating of the tooth, the tolerance of E. faecalis to pulsed heating in sub-second time range should be determined. With regard to low temperature rise detected in a previous study [23], which cannot be responsible for bactericidal effects, there is a possibility of the existence of a previously mentioned unidentified photodamage effect [27, 28], possibly dependent on the microenvironment of infected dentinal tubules. Colonization and degradation of dentin matrix due to bacterial action also changes optical properties of dentin resulting in the increase of the absorption of NIR wavelengths. Infected areas of dentin could be potential selective targets for NIR wavelengths, opening the potential to achieve localized hotspots within dentin, causing thermal damage to limited areas of dentin without causing bulk overheating of the tooth. Studies of Nd:YAG irradiation of carious and healthy dentin [30] demonstrate a substantially higher absorption of 1,064-nm wavelength in carious dentin in comparison to healthy dentin. We also performed a basic thermal measurement with the use of a thermal camera comparing the temperature rise on the surface of carious and healthy dentin which were heated in non-contact mode by a Nd:YAG laser. We determined that ∆T for carious dentin is in the range of 3.4 times higher per J/cm2 than for healthy dentin (unpublished data). The results seem to confirm that dentin, which had its optical properties changed by bacterial action, could represent a selective target for NIR laser heating due to different optical properties which lead to higher absorption. This would also seem to explain the lethal effect of NIR lasers in root canals, where heating of the bacterial microenvironment seems to be one of the primary reasons for the lasers' effectivity, especially where nonpigmented bacteria are targeted. Further optimization of irradiation parameters should improve the overall success rate of laser-assisted endodontic treatment. New studies targeted towards analyzing the potential photodamage effect on a sub-cellular level, as well as studies aimed at analyzing the spot and area temperature rises with the help of thermal imaging in sound and infected dentin, could help to optimize the already established procedure.
Conclusions
A novel method using a highly transparent substrate in the NIR spectrum, sapphire, was used to determine the direct effect of two popular wavelengths used in laser-assisted endodontics (808-nm and 1,064-nm) on pigmented and unpigmented bacteria. The 808-nm and 1,064-nm lasers, used at standard parameters for laser-assisted endodontic procedures [14, 23, 31], have very limited direct bactericidal effect on unpigmented bacteria regardless of their cell wall structure (i.e., Gram-positive and Gram-negative bacteria) and a moderate direct bactericidal effect on black pigmented P. gingivalis. The most likely mechanism explaining the lethal effect of 808-nm and 1,064-nm lasers [14, 20, 23] in laser-assisted endodontics, at least for unpigmented bacteria, would appear to be laser-induced, short-term localized heating of the bacterial microenvironment to lethal temperatures. Our research suggests that thorough understanding of combined optical characteristics of both the bacteria and their microenvironment plays a key role in devising optimal parameters for the laser-assisted endodontic procedure.
References
Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G (1990) Intraradicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod 16:580–588
Berutti E, Marini R, Angeretti A (1997) Penetration ability of different irrigants into dentinal tubules. J Endod 23:725–727
Kouchi Y, Ninomiya J, Yasuda H, Fukui K, Moriyama T, Okamoto H (1980) Location of Streptococcus mutans in the dentinal tubules of open infected root canals. J Dent Res 59:2038–2046
Estrela C, Estrela CRA, Decurcio DA, Hollanda ACB, Silva JA (2007) Antimicrobial efficacy of ozonated water, gaseous ozone, sodium hipochlorite and chlorhexidine in infected human root canals. Int Endod J 40:85–93
Spratt DA, Pratten J, Wilson M, Gulabivala K (2001) An in vitro evaluation of the antimicrobial efficacy of irrigants on biofilms of root canal isolates. Int Endod J 34:300–307
Noiri Y, Ehara A, Kawahara T, Takemura N, Ebisu S (2002) Participation of bacterial biofilms in refractory and chronic periapical periodontitis. J Endod 28:679–683
Distel JW, Hatton JF, Gillespie MJ (2002) Biofilm formation in medicated root canals. J Endod 28:689–693
George S, Kishen A, Song KP (2005) The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod 31:867–872
Bale MJ, Bennett PM, Beringer JE, Hinton M (1993) The survival of bacteria exposed to desiccation on surfaces associated with farm buildings. J Appl Bacteriol 75:519–528
Tendolkar PM, Baghdayan AS, Shankar N (2003) Pathogenic enterococci: new developments in the 21st century. Cell Mol Life Sci 60:2622–2636
Hartke A, Giard JC, Laplace JM, Auffray Y (1998) Survival of Enterococcus faecalis in an oligotrophic microcosm: changes in morphology, development of general stress resistance, and analysis of protein synthesis. Appl Environ Microbiol 64:4238–4245
Siqueira JF Jr, Rôças IN (2005) Exploiting molecular methods to explore endodontic infections: part 2–redefining the endodontic microbiota. J Endod 31:488–498
Vaarkamp J, ten Bosch JJ, Verdonschot EH (1995) Propagation of light through human dental enamel and dentine. Caries Res 29:8–13
Klinke T, Klimm W, Gutknecht N (1997) Antibacterial effects of Nd:YAG laser irradiation within root canal dentine. J Clin Laser Med Surg 15:29–31
Odor TM, Chandler NP, Watson TF, Ford TR, McDonald F (1999) Laser light transmission in teeth: a study of the patterns in different species. Int Endod J 32:296–302
Myers TD, McDaniel JD (1991) The pulsed Nd:YAG laser: review of clinical applications. J Calif Dent Assoc 19:25–30
Hardee MW, Miserendino L, Kos W, Walia H (1994) Evaluation of the antibacterial effects of intracanal Nd:YAG laser irradiation. J Endod 20:377–380
Hassan FE (1995) A new method for treating weeping canals: clinical and histopathologic study. Egypt Dent J 41:1403–1408
Moritz A, Gutknecht N, Schoop U, Goharkhay K, Doertbudak O, Sperr W (1997) Irradiation of infected root canals with a diode laser in vivo: results of microbiological examinations. Lasers Surg Med 21:221–226
Moritz A, Gutknecht N, Goharkhay K, Schoop U, Wernisch J, Sperr W (1997) In vitro irradiation of infected root canals with a diode laser: results of microbiologic, infrared spectrometric, and stain penetration examinations. Quintessence Int 28:205–209
Gutknecht N, van Gogswaardt D, Conrads G, Apel C, Schubert C, Lampert F (2000) Diode laser radiation and its bactericidal effect in root canal wall dentin. J Clin Laser Med Surg 18:57–60
Kreisler M, Kohnen W, Beck M, Al Haj H, Christoffers AB, Gotz H, Duschner H, Jansen B, D’Hoedt B (2003) Efficacy of NaOCl/H2O2 irrigation and GaAlAs laser in decontamination of root canals in vitro. Lasers Surg Med 32:189–196
Schoop U, Kluger W, Moritz A, Nedjelik N, Georgopoulos A, Sperr W (2004) Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med 35:111–116
Bergmans L, Moisiadis P, Teughels W, Van Meerbeek B, Quirynen M, Lambrechts P (2006) Bactericidal effect of Nd:YAG laser irradiation on some endodontic pathogens ex vivo. Int Endod J 39:547–557
Wang QQ, Zhang CF, Yin XZ (2007) Evaluation of the bactericidal effect of Er, Cr:YSGG, and Nd:YAG lasers in experimentally infected root canals. J Endod 33:830–832
Alsharif R, Godfrey W (2002) Bacterial detection and live/dead discrimination by flow cytometry. Microbial Cytometry Application Note. BD Biosciences, Immunocytometry Systems; San Jose, CA.
Neuman KC, Chadd EH, Liou GF, Bergman K, Block SM (1999) Characterization of photodamage to Escherichia coli in optical traps. Biophys J 77:2856–2863
Mirsaidov U, Timp W, Timp K, Mir M, Matsudaira P, Timp G (2008) Optimal optical trap for bacterial viability. Phys Rev E Stat Nonlin Soft Matter Phys 78(2 Pt 1):021910
Murray BE (1990) The life and times of the Enterococcus. Clin Microbiol Rev 3:46–65
Jalil LA, Labella R, Pearson GJ (1997) Surface topography of enamel and dentine from primary teeth following infra-red Nd-YAG laser irradiation: an in vitro study. Lasers Med Sci 12:61–67
Gutknecht N (2004) Der Fidelis Plus Laser von Fotona. Zahnaertztliche Indikationen, Parameter und Behandlungsmassnahmen. In: Endodontie, Laser Franz Medien GmbH, Germany, p. 26
Acknowledgements
We thank Dr. K. Seme from the Institute of Microbiology and Immunology, Faculty of Medicine for kindly providing the bacterial strains.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pirnat, S., Lukac, M. & Ihan, A. Study of the direct bactericidal effect of Nd:YAG and diode laser parameters used in endodontics on pigmented and nonpigmented bacteria. Lasers Med Sci 26, 755–761 (2011). https://doi.org/10.1007/s10103-010-0808-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-010-0808-7