Abstract
The capacity of photo-sensitizers, used in combination with laser light to kill micro-organisms has been demonstrated in different studies. Photo-activated disinfection (PAD) has been introduced in periodontology as an aid for disinfection of periodontal pockets. The aim of this study is to verify the harm for dental vitality of the use of PAD in periodontal pockets. Root canals of 24 freshly extracted human teeth where prepared using profiles up to a size of ISO #50 and filled with thermo-conductor paste. A silicon-based false gum was made in which a periodontal pocket was created and filled with photo-sensitizer phenothiazine chloride (phenothiazine-5-ium, 3.7-bis (dimethylamino)-, chloride). The external root surface was irradiated during 60 s with a 660-nm diode laser (output power: 20 mW; power density: 0.090 W/cm2; Energy density: 5.46 J/cm2) using a periodontal tip with a diameter of 1 mm and a length of 7 mm. Temperatures were recorded inside the root canal using a thermocouple. Measurements were recorded every second, starting at 10 s before lasering, during the irradiation and were continued for 150 s after the end of irradiation, and six measurements were done per tooth. An average temperature increase of 0.48 ± 0.11°C was recorded. Our results demonstrated that pulp temperature increase was lower than 3°C, which is considered to be harmless for pulp injury. Regarding pulp temperature increase, the use of PAD for disinfection of periodontal pockets can be considered as a safe procedure for dental vitality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal diseases affect around 30% of the adult population. These diseases occur due to the presence of pathogen germs (Porphyromonas gingivalis, Actinobacillus actinomycetemcomitans), which induce inflammation of periodontal tissues and resorption of the alveolar bone [1, 2].
The reduction of the pathogen bacterial flora is done classically by mechanical debridement (root shaving) in conjunction with an antimicrobial treatment by systemic way [3, 4].
The complete elimination of the biofilm and layers of sediment in periodontal pockets is very difficult to obtain after treatment, particularly in the deepest level of pockets [5–8].
An adjunctive treatment for the mechanical debridement is the disinfection by photo-activation. This device is a laser diode with an emission of visible red color, a wavelength of 635 nm and of a photo-sensitizer: toluidine blue [9].
The application of a high concentration of toluidine blue (during PAD) is not toxic to humans [10, 11] in endodontic nor in the treatment of decays [12].
Studies conducted on animals also confirm that when PAD is applied to the periodontal pockets there are no toxic effects on these tissues either [13].
However, the efficiency of the decontamination of the periodontal pockets by PAD has not yet been completely clarified [14, 15]. The aim of this in vitro study is therefore to evaluate the pulp temperature increase during the application of the PAD for periodontal pocket decontamination.
Materials and methods
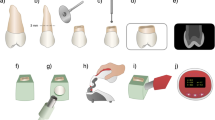
Preparation of teeth
Twenty-four freshly extracted, caries-free, single-rooted, human teeth were used. They where stored in a 0.05% NaOCl solution. The roots were cut at a length of approximately 10 mm. Root canals were prepared up to an apical size ISO #50 using ProFile instruments (Dentsply Maillefer, Baillaigues, Switzerland) by means of a low-torque control motor (ATR technika torque control, ATR, Pistoia, Italy) in order to be able to insert the thermocouple probe (Ø = 400 µ) into the root canal.
The teeth were putted in silicon-based false gum, in which a periodontal pocket was created and filled with photosensitizer phenothiazine chloride (phenothiazine-5-ium, 3.7-bis (dimethylamino)-, chloride) (HELBO blue photosensitizer, HELBO photodynamic systems GmbH & Co. KG, Walldorf, Germany)
Pocket creation
Wax was applied on the root surfaces from the cement–enamel junction (CEJ) of every tooth at a distance of 1.5 mm from the apex.
Each waxed tooth was pushed into a silicon paste. After the polymerization of the silicon, the tooth was rinsed with hot water with the aim to remove the wax and to create the pocket between the tooth surface and the silicon paste.
Protocol for photo-activated disinfection
For the PAD, we used a phenothiazine chloride solution (phenothiazine-5-ium, 3.7-bis (dimethylamino)-, chloride: 1 ml contains: 10 mg phenothiazine-5-ium, 3.7- bis (dimethylamino)-, chloride buffered to pH = 3.5 with citrate buffer system, isotonized and viscosity-optimized. with 1% HPMC) (HELBO blue photosensitizer, HELBO photodynamic systems GmbH & Co. KG, Walldorf, Germany).
Laser device
A 660-nm diode laser with a power output of 20 mW (TheraLite laser; HELBO photodynamic systems GmbH & Co. KG, Walldorf, Germany) was used. This is a class 2M laser device (output power 20 mW; continuous wave; optical fiber). The laser tip fiber is 7 mm in length and 1 mm in diameter (HELBO 3D pocket probe, HELBO photodynamic systems GmbH & Co. KG, Walldorf, Germany). The fiber was inserted into the artificial pocket parallel and close to the external root surface. Then, the laser fiber was kept stationary by a mechanical holder during temperature measurement. The laser fiber emitted irradiation only from lateral sides (Schema 1). Dye solution was irradiated following the manufacturer's recommendations for 60 s at 20-mW output power in a continuous wave. Energy density delivered to the photosensitizer was 5.46 J/cm2. The power density was: 0.090 W/cm2.
Temperature measurement
Thermo-conductor paste
A thermo-conductor paste (warmeleitpaste WPN 10, Austerlitz Electronic, Nürnberg, Germany) was injected by Lentulo into the root canals to ensure good thermal contact between the root walls and the thermocouple. The thermal conductivity of the paste is 1.674 J/ s−1 m−1 K−1. This is comparable to the thermal conductivity of soft tissues (0.837–2.093 J/ s−1 m−1 K −1 depending on hydration).
Thermocouple
A type K thermocouple was used (Cr-Al) with a Picolog TC08 temperature-recording device (Pico Technology). The accuracy is 0.01°C. One thermocouple was placed in the root canal at 1 mm from the apical part of the root (Schema 1). A second thermocouple was placed at room temperature to compare temperature changes at the root surface with changes in room temperature. Laser irradiation was started when the temperature at the root surface was approximately constant and similar to the room temperature. Temperatures were recorded every second starting at 10 s before irradiation, during irradiation, and for 150 s after the end of irradiation. The considered temperatures (Δt) were calculated as the difference between recorded temperatures at root surface (TRS) and recorded room temperatures (TRT): Δt = TRS - TRT.
The mean of the recorded temperatures (Δt) and standard deviation were calculated. Normality tests were done using the Kolmogorov and Smirnov method.
Results
After 150 s of irradiation, the mean and standard deviation of temperature increase at root canals were 0.48 ± 0.11°C (Graph 1).
The average of the minimum of temperature increase was 0.26 ± 0.07°C. The maximum of temperature increase was 0.79 ± 0.04°C in average. Temperature increases in all recordings were below the safety level of 3°C (16) for pulp injury.
Graph 2 shows an example of six records of temperature rise. The data passed the normality test (KS) with P-value > 010.
Our in vitro results demonstrate that the use of the PAD for periodontal pocket decontamination can be considered as a harmless technique for pulp vitality.
Discussion
Numerous studies have been conducted to determine not only the most efficient photo-sensitizer for killing specific oral pathogens but also the most efficient combination of photo-sensitizer and laser light. Photosensitizers have been shown to be effective against a wide range of Gram-positive and Gram-negative bacteria [17]. A key property of photosensitizers is that they should absorb laser light in order to generate a therapeutic effect. The red visible wavelengths give the greatest penetration of tissues surrounding a wound or lesion, and will also penetrate any blood that may be present.
There is considerable interest in the use of locally applied antimicrobial agents in periodontal treatment [18]. However, the reduced time of presence of antimicrobial agents in topical application leads to a decrease in their effectiveness [19].
Bacteria included in the biofilms are tens to a thousand times less sensitive to antibiotics than those bacteria in suspension due to the difficulty of diffusion of drugs [20] and the possibility of phenotypic adaptation of some bacteria could help to explain these therapeutic failures. Currently, the importance of dispersion of biofilm is considered to be the key for an effective therapeutic approach. The PAD seems to be a good alternative to these topical agents in periodontal decontamination in view of its very significant action on Porphyromonas gingivalis (study on rats) [21], and on bacteria present in the oral biofilm model [22].
Today, the importance of the dispersal biofilm is considered as the key to an effective therapeutic approach.
In terms of bacteria associated with periodontitis, several photo-sensitizers have been shown to be effective when trialed in laboratory models [17–20, 23–29].
Typical target bacterial species for these studies have been comprised of Porphyromonas gingivalis , Actinobacillus actinomycetemcomitans, and fuso bacterium nucleatum [30, 31];
Effective killing by PAD (with reductions in viability of (97.2%, 99.9%, and 99.4%, respectively, have been obtained using tolonium chloride (25 µg/mL), although methylene blue and ADP give somewhat less uniform results across these species [32].
PAD seems to be a good alternative to these topical agents in the periodontal decontamination in view of its very significant action on Porphyromonas gingivalis (study on rats) [21], and on bacteria present in the oral biofilm model [22].
PAD is a novel form of low-power laser therapy in which the laser energy in itself is not particularly lethal to bacteria, but is used to achieve photochemical activation of oxygen-releasing dyes. Singlet oxygen released from the dyes causes membrane and DNA damage to microorganisms, thereby leading to their rapid death [33].
The effectiveness of PAD is influenced by the type and concentration of dye, the laser parameters employed, and the local growth environment (biofilm and growth state) [33].
It is important to mention that the side effects like diffusion of the dye towards the apex and its consequences were not considered in this study.
On the other hand, before starting any possible clinical study about applications of the PAD in periodontics, it was necessary to verify the harm risks of this PAD technique on pulp vitality of vital teeth.
Zach and Cohen had demonstrated that to cause a pulpal protein denaturation, the pulpal temperature increase must be lower than 3ºC to avoid pulp damage [16]. The results of our in vitro study showed that the increase in pulp temperature was 0.48ºC ± 0.11. Thus, based on our results, it can be considered that the use of the PAD in periodontal pockets for decontamination purpose is harmless to pulp vitality.
On the other hand, it is important to mention that the artificial model of pocket used in this study cannot totally represent the periodontal pocket in vivo. This artificial model can certainly have some limits. Future in vivo studies should be conducted to confirm the safety of this PAD technique in periodontal applications and to detect any eventual side effects of this technique on dental or periodontal tissues.
Conclusions
The use of PAD for periodontal pocket decontamination can be considered a safe and effective technique for maintaining pulp vitality with regards to the irradiation conditions used in this in vitro study.
References
Manson JD, Eley BP (1995) Outline of periodontics, 3rd edn. Wright, Oxford, UK
Miyazaki H, Pilot T, Leclercq MH, Barmes DE (1991) Profiles of periodontal conditions in adults measured by CPTIN. Int Dent J 41(2):67–73
Feres M, Haffajee AD, Goncalves C, Allard KA, Som S, Smith C, Goodson JM, Socransky SS (1999) Systemic doxycycline administration in the treatment of periodontal infection. II. Effect on antibiotic resistance of subgingival species. J Clin Periodontol 26:784–792
Chaves ES, Jeffcoat MK, Ryerson CC, Snyder B (2000) Persistent bacterial colonisation of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J Clin Periodontol 27:897–903. doi:10.1034/j.1600-051x.2000.027012897.x
Walker A, Ash MN (1976) A study of root planning by scanning electron microscopy. Dent Hyg (Chic) 50:109–114
Waerhang J (1978) Healing of dento-epithelial junction following subgingival plaque control. II; As observed on extracted teeth. J Periodontol 49:119–134
Cafesse RG, Sweeney PL, Smith BA (1986) Scaling and root planning with and without periodontal flap surgery. J Clin Periodontol 13:205–210. doi:10.1111/j.1600-051X.1986.tb01461.x
Buchanan SA, Robertson PB (1987) Calculus removal by scaling and root planning with and without surgical access. J Periodontol 58:159–163
Lee MT (2003) Photoactivated disinfection of E. Faecalis in root canals using lasers. The University of Queensland, MDSc. St. Lucia
Sarkar S, Wilson M (1993) Lethal photosensitisation of bacteria in subgingival plaque from patients with chronic periodontitis. J Periodont Res 28:204–210
Haas R, Baron M, Dortbudak O, Watzek G (2000) Lethal photosensitisation, autologous bone and e-PTFE membrane for the treatment of peri-implantitis: preliminary results. Laryngoscope 105:867–871
Odor TM, Chandler NP, Watson TF, Pitt Ford TR, McDonald F (1999) Laser light transmission in teeth: a study of the patterns in different species. Int Endodontic J 32:296–302
Komerik N, Wilson M (2002) Factors influencing the susceptibility of Gram-negative bacteria to toluidine blue-mediated lethal photosensitisation. J Appl Microbiol 92:618–623. doi:10.1046/j.1365-2672.2002.01567.x
Braun A, Dehn C, Krause F, Jepsen S (2008) Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: a randomized clinical trial. J Clin Periodontol Oct 35(10):877–884
Christodoulides N, Nikolidakis D, Chondros P, Becker J, Schwarz F, Rössler R, Sculean A (2008) Photodynamic therapy as an adjunct to non-surgical periodontal treatment: a randomized controlled clinical trial. J Periodontol 79(9):1638–1644
Zach L, Cohen G (1965) Pulp response to externally applied heat. Oral Surg Oral Med Oral Pathol 19:515–530. doi:10.1016/0030-4220(65)90015-0
Slots J, Jorgensen MJ (2002) Effective, safe, practical and affordable periodontal antimicrobial therapy: where are we going, and are we there yet? J Periodontol 2000(28):298–312
Oosterwaal PJ, Mikx FH, Renggli HH (1990) Clearance of a topically applied fluorescein gel from periodontal pockets. J Clin Periodontol 17:613–615. doi:10.1111/j.1600-051X.1990.tb01681.x
Marsh P (2003) Plaque as a biofilm: pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis 9(Suppl 1):16–22. doi:10.1034/j.1601-0825.9.s1.4.x
Bhatti M, MacRobert A, Meghji S, Henderson B, Wilson M (1998) A study of the uptake of toluidine blue O by Porphyromanos gingivalis and the mechanism of lethal photosensitization. Photochem Photobiol 68:370–376. doi:10.1111/j.1751-1097.1998.tb09694.x
Bhatti M, Macrobert A, Henderson B, Shepherd P, Cridland J, Wilson M (2000) Antibody targeted lethal photosensitization of Porphyromonas gingivalis. Antimicrob Agents Chemother 44:2615–2618. doi:10.1128/AAC.44.10.2615-2618.2000
Komerik N, Wilson M, Pool S (2000) The effect of photodynamic action on two virulence factors of Gram-negative bacteria 72:676–680
Komerik N, Nakanishi H, Macrobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47:932–940. doi:10.1128/AAC.47.3.932-940.2003
Sarkar S, Wilson M (1993) Lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. J Periodontal Res 28:204–210. doi:10.1111/j.1600-0765.1993.tb01070.x
Wilson M, Dobson J, Sarkar S (1993) Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 8:182–187. doi:10.1111/j.1399-302X.1993.tb00663.x
Wilson M, Dobson J (1993) Lethal photosensitization of oral anaerobic bacteria. Clin Infect Dis 16(suppl 4):S414–S415
Wilson M, Sarkar S, Bulman JS (1993) Effect of blood on lethal photosensitization of bacteria in subgingival plaque from patients with chronic periodontitis. Lasers Med Sci 8:297–303. doi:10.1007/BF02547854
Wilson M (1994) Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J 44:181–189
de Oliveira RR, Schwartz-Filho HO, Novaes AB Jr., Taba M Jr. (2007) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontol 78(6):965–973. doi:10.1902/jop. 2007.060494
Komerik N, Curnow A, Macrobert AJ, Hopper C, Speight PM, Wilson M (2002) Fluorescence biodistribution and photosensitizing activity of toluidine blue O on rat buccal mucosa. Lasers Med Sci 17:86–92. doi:10.1007/s101030200015
O’Neill JF, Hope CK, Wilson M (2002) Oral bacteria in multi-species biofilms can be killed by red light in the presence of toluidine blue. Lasers Surg Med 31:86–90. doi:10.1002/lsm.10087
Wilson M, Wilson H (1997) Laser treatment, US patent 5,611,793
Walsh LJ (2003) The current status of laser applications in dentistry. Aust Dent J 48:146–155. doi:10.1111/j.1834-7819.2003.tb00025.x
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Yazami, H., Zeinoun, T., Bou Saba, S. et al. Pulp temperature increase during photo-activated disinfection (PAD) of periodontal pockets: an in vitro study. Lasers Med Sci 25, 655–659 (2010). https://doi.org/10.1007/s10103-009-0686-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0686-z