Abstract
The study objective was to evaluate the effects of photodynamic therapy on buccal candidiasis in rats. After experimental candidiasis had been induced on the tongue dorsum, 72 rats were distributed into four groups according to treatment: treated with laser and methylene blue photosensitizer (L+P+); treated only with laser (L+P−); treated only with photosensitizer (L−-P+); not treated with laser or photosensitizer (L−P−). The rats were killed immediately, 1 day, or 5 days after treatment, for microscopic analysis of the tongue dorsum. Observation verified that the photodynamic therapy group (L+P+) exhibited fewer epithelial alterations and a lower chronic inflammatory response than the L−P− group. The group L+P− presented more intense epithelial alterations and chronic inflammatory response than the remaining groups. The L−P+ group showed tissue lesions similar to those of the L−P− group. In conclusion, rats treated with photodynamic therapy developed more discrete candidiasis lesions than did the remaining groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida albicans is the causative agent of numerous diseases that vary from superficial mucosa infections, such as vulvovaginal candidiasis and oropharyngeal candidiasis, to potentially fatal systemic diseases. Oropharyngeal candidiasis is very common in patients with acquired immunodeficiency syndrome (AIDS), while deep systemic infections are frequently associated with neutropenia, resulting from antineoplastic therapy or immunosuppressive therapy after organ transplantation [1].
The treatment of candidiasis with antifungal agents such as polyenes (nystatin and amphotericin B) or clotrimazole only results in transitory improvement of candidiasis, with recurrence a common problem, especially in cases of immunodeficiency. More promising results are obtained with systemic antifungals, such as ketoconazole, fluconazole and itroconazole; however, the use of these medications provokes side effects and the development of microbial resistance [2].
Because of this situation, the development of alternative therapies for the treatment of candidiasis, such as photodynamic therapy, is fundamental [2–4]. Photodynamic therapy (PDT) is a relatively new modality in cancer treatment that is being investigated in the area of medicine worldwide [5, 6]. PDT application in dentistry is growing rapidly in the treatment of oral cancer, bacterial and fungal infections, and in the diagnosis of the malignant transformation of oral lesions. PDT has shown potential in the treatment of oral leukoplakia, oral lichen planus and head and neck cancer [7].
In PDT a photosensitizer is activated by exposure to light of a specific wavelength in the presence of oxygen. The energy transfer from the activated photosensitizer to the available oxygen forms toxic oxygen species, such as singlet oxygen and free radicals. These reactive chemical species cause the destruction of proteins, lipids, nucleic acids and other cellular components. PDT presents no genotoxic or mutagenic effects, which impedes the development of microbial resistance [4, 7, 8].
The efficacy of PDT on the Candida genus yeasts has been demonstrated by several in vitro studies [2, 9–11]. Wilson and Mia [2] irradiated C. albicans suspensions with helium–neon or gallium arsenate–aluminum lasers in association with different photosensitizers: toluidine blue, methylene blue, tionin, violet crystal and phthalocyanine. Except for phthalocyanine, all other photosensitizers tested caused a significant reduction in C. albicans count after PDT.
Chabrier-Roselló et al. [9] verified that C. albicans metabolic activity in the biofilm was diminished after application of PDT with photofrin at 10 μg/ml for 30 min when compared with treatment with amphotericin B (10 μg/ml) for the same period.
Souza and colleagues [10] observed that laser radiation in the presence of methylene blue was able to reduce the number of Candida genus yeasts, with an 88.6% reduction for C. albicans, 84.8% for C. dubliniensis, 91.6% for C. krusei and 82.3% for C. tropicalis. Munin and co-workers [11] verified that PDT with methylene blue was able to inhibit the formation of the germinative tubes of C. albicans and concluded that this therapy affected the pathogenicity of yeasts of the Candida genus.
Despite the promising results of the work realized in vitro, few studies have focused on the effects of PDT in experimental models. Teichert et al. [3] tested several concentrations of methylene blue associated with a diode laser on buccal candidiasis in immunosuppressed mice. Analysis of the results indicated the efficacy of PDT at reducing yeasts, an effect that was directly proportional to the photosensitizer concentration.
Thus, the objective of this work was to evaluate the effects of photodynamic therapy on buccal candidiasis in rats.
Materials and methods
Experimental animals
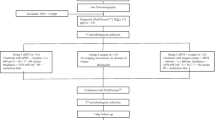
The study was approved by the Research Ethics Committee of the São José dos Campos School of Dentistry (UNESP), under protocol number 008/2005-PACEP. Seventy-two rats (Rattus norvegicus, Albinus, Wistar) with no Candida genus in the buccal cavity, weighing approximately 250 g, were included in the study.
Induction of experimental candidiasis
The rats were given a 0.1% solution of tetracycline hydrochloride (Terramycin, Pfizer, São Paulo, Brazil) in their drinking water. This treatment was initiated 7 days prior to the inoculation of a C. albicans suspension and was maintained up to the end of the experiment [12].
A suspension of C. albicans containing 5 × 108 viable cells/ml was prepared according to Reed et al. [13]. A strain isolated from a patient with denture stomatitis was used. For the inoculation of this suspension, the rats were sedated via intramuscular injection of xylazine chloride solution (Bayer, São Paulo, Brazil) and ketamine (Virbac, São Paulo, Brazil) in the proportion 1/0.5 ml, at a dose of 0.05 ml/100 g of body weight. The C. albicans suspension (0.2 ml) was dripped into the mouths of the rats with a 1 ml syringe. Next, the material was spread on the tongue dorsum with a swab previously soaked in the suspension. This procedure was repeated for three consecutive days.
Photodynamic therapy
Seven days after the final C. albicans inoculation, the rats were distributed into four experimental groups (n = 18) according to the following treatments: photodynamic therapy with methylene blue and laser (L+P+); laser treatment and physiological solution (L+P−); treatment with photosensitizer in the absence of laser (L−P+); and no treatment, just physiological solution alone (L−P−).
A 0.1 mg/ml solution of methylene blue was used as the photosensitizer, which we prepared by dissolving the powder (Labsynth, São Paulo, Brazil) in a physiological solution of 0.85% sodium chloride (NaCl). Next, the solution was filtered through a sterilized membrane with 0.22 μm diameter pores (TPP, Trasadingen, Switzerland). A gallium–aluminum–arsenide laser (Easy Laser, Clean Line, São Paulo, Brazil) was used at a wavelength of 660 nm, 50 mW power, 10 J energy and an energy density of 26 J/cm2.
To perform the treatment, we anesthetized the rats via intramuscular injection of xylazine chloride solution (Bayer) and ketamine (Virbac) in the proportion 1/0.5 ml, at a dose of 0.1 ml/100 g of body weight. Next, the methylene blue solution was applied topically to the tongue dorsum with a sterilized swab. After 5 min (pre-irradiation time), two areas on the tongue were irradiated by laser, one on the anterior portion of the tongue and the other in the region of the giant papillae. The irradiation time for each application was 200 s (L+P+ group). The effect of photosensitizer only was tested by application of the methylene blue solution for the same period as that for the L+P+ group, without the laser treatment (L−P+ group). To verify the effect of the laser only, we substituted the methylene blue solution with physiological solution, and laser application was the same as for the L+P+ group (L+P− group). The fourth group of rats were given only physiological solution, with no laser treatment (L−P− group).
Euthanasia of the rats
In each experimental group, the rats were killed immediately (n = 6), 24 h (n = 6) or 5 days (n = 6) after their respective treatments, corresponding to 7 days, 8 days and 12 days after the induction of candidiasis. Their tongues were removed and examined by stereomicroscopy.
Microscopic analysis
For light microscopy analysis, the tongues were fixed in 10% formalin for 24 h and hemisected in the sagittal plane. Next, the tissue samples were mounted in paraffin, and sections 7 μm thick were stained with hematoxylin–eosin (H&E) and periodic acid–Schiff (PAS).
We performed semiquantitative analyses to assess the degree of epithelium colonization by Candida spp., based on the methodology proposed by Junqueira et al. [14]. Twenty-eight histological fields of the tongue dorsum in each section, in the anteroposterior direction, were examined with a ×40 objective lens and a ×10 ocular lens (Carl Zeiss, Jena, Germany). A score ranging from 0 to 4 was given for each histological field: 0, absence of colonization; 1, one to five hyphae; 2, six to 15 hyphae; 3, 16 to 50 hyphae; 4, more than 50 hyphae. Two randomly selected histological sections were examined from each rat for a total of 56 scores per rat. For statistical analysis, the average score was determined from the 56 scores.
The intensity of the tissue lesions was evaluated by the attribution of scores for epithelial alterations and the inflammatory response of the conjunctive tissue.
For the epithelial tissue, seven tissue alterations were analyzed: epithelial hyperplasia, disorganization of the basal layer, exocytosis, spongiosis, loss of filiform papillae, hyperparakeratosis, and the formation of intra-epithelial micro-abscesses. Each epithelial alteration was classified as absent, discrete, or accentuated, such that the scores attributed were: 0 (absence of epithelial lesion), 1 (discrete epithelial lesion) and 2 (accentuated epithelial lesion), with each rat tongue dorsum receiving a score varying from 0 to 14 points.
In relation to chronic inflammatory infiltrate of the conjunctive tissue, the following scores were attributed: 0 (absence of inflammatory cells), 1 (discrete inflammatory infiltrate) and 2 (accentuated inflammatory infiltrate).
Statistical analysis
We used Kruskal–Wallis and Bonferroni tests to determine significance between the different groups, considering a 5% level of significance (P < 0.05).
Results
During macroscopic examination, clinical candidiasis lesions were observed for all the euthanasia periods. These lesions were characterized by areas of localized papillary atrophy, principally in the region of the giant papillae. The number of rats that presented clinical lesions on the tongue dorsum is described in Table 1.
Microscopic examination of the tongue dorsum verified that the majority of the rats no longer showed Candida colonization. In the rat tongues that still presented the microorganism, few hyphae were observed on the epithelium surface, with no statistically significant differences between the groups (Table 2).
Intense tissue lesions were observed, characterized by numerous epithelial lesions, including epithelial hyperplasia, disorganization of the basal layer, exocytosis, spongiosis, increased mitoses numbers in the basal layer, loss of filiform papillae, and hyperparakeratosis. In some rat tongues, the presence of intra-epithelial micro-abscesses was also verified. In these areas of tissue lesions, the lamina propria exhibited predominately mononuclear inflammatory infiltrate and, occasionally, congested vessels. In certain histological sections, polymorphonuclear inflammatory infiltrate was also observed, characterized by neutrophils. In the majority of rat tongues, the tissue lesions were located at the transition between simple conical and giant papillae; although some tongues also exhibited lesions in the region of the true papillae.
For the rats that were killed immediately, no statistically significant differences were observed between the groups L+P+, L+P−, L−P+ and L−P−, for either epithelial alterations (P = 0.227) or inflammatory response of the lamina propria (P = 0.465). However, for rats killed after 1 day and after 5 days, differences were observed between the groups (Kruskal-−Wallis test), for either epithelial alterations (1 day, P = 0.013 and 5 days, P = 0.028) or inflammatory response of the lamina propria (1 day, P = 0.004 and 5 days, P = 0.005). The group that was given PDT (L+P+) exhibited fewer epithelial alterations and a lower chronic inflammatory response than the control group (L−P−). The group treated only with the laser (L+P−) presented more intense candidiasis lesions than the remaining groups, with a statistically significant difference between the L+P− and L+P+ groups (Bonferroni test) for either epithelial lesions (1 day, P = 0.0013 and 5 days, P = 0.0030) and chronic inflammatory response (1 day, P = 0.0016 and 5 days, P = 0.0003). In the L+P− group, large intra-epithelial abscesses were observed and numerous neutrophils in the conjunctive tissue. The group treated only with the photosensitizer (L−P+) revealed tissue lesions similar to the those in the control group (L−P−) (Table 2).
Figures 1, 2 and 3 show characteristic tissues lesions for the L+P+, L+P−, L−P+ and L−P− groups, respectively, for the euthanasia times immediately, 1 day, and 5 days.
Killed immediately, region of giant papillae. a Tissue lesion with epithelial hyperplasia, disorganized basal layer, exocytosis and mononuclear inflammatory infiltrate in the lamina propria. b, c, d Tissue lesions, characterized by epithelial hyperplasia, disorganized basal layer, exocytosis, spongiosis, loss of filiform papillae, hyperparakeratosis and inflammatory infiltrate. The lamina propria presents a mononuclear inflammatory infiltrate. H&E, ×200
Killed after 1 day, region of giant papillae. a Discrete mononuclear inflammatory infiltrate in the lamina propria. b Intense tissue lesion, characterized by epithelial hyperplasia, disorganized basal layer, exocytosis, loss of filiform papillae, hyperparakeratosis and extensive intra-epithelial abscess. c, d Tissue lesions, characterized by epithelial hyperplasia, disorganized basal layer, exocytosis, spongiosis, loss of filiform papillae, hyperparakeratosis. The lamina propria presents mononuclear inflammatory infiltrate. H&E, a,b ×100 and c,d ×200
Killed after 5 days, region of giant papillae. a Discrete mononuclear inflammatory infiltrate in the lamina propria. b Intense tissue lesion, characterized by epithelial hyperplasia, disorganized basal layer, exocytosis, loss of filiform papillae, hyperparakeratosis and intra-epithelial abscess. c, d tissue lesions with epithelial hyperplasia, disorganized basal layer, exocytosis, spongiosis, loss of filiform papillae, hyperparakeratosis. The lamina propria presents mononuclear inflammatory cells. H&E, ×200
Discussion
Several in vitro studies have demonstrated that photodynamic therapy has lethal effects on yeasts of the Candida genus [2, 10, 11, 15]. However, few studies on PDT that involve animal models have been realized [4]. The development of in vivo research is extremely important, since the action of PDT on microorganisms could be affected by the environmental conditions of the oral cavity, including the presence of saliva, pH variations, mucosa characteristics, and the action of the immunological system [16].
The percentage of rats showing macroscopic lesions on the tongue dorsum was between 37.5 and 45.8, according to the euthanasia time, which varied from 7 days to 12 days after candidiasis had been induced. These results are in agreement with those of Yujra et al. [17], who observed clinical candidiasis lesions in 50% of rats killed 7 days after C. albicans inoculation. In contrast, Junqueira et al. [14] verified macroscopic lesions on the tongue dorsum in 66% and 75% of rats 7 days and 15 days after candidiasis induction, respectively.
One day and 5 days after the experimental treatment, the group that underwent PDT (L+P+) exhibited no clinical candidiasis lesions, while the remaining groups (L+P−, L−P+, L−P−) all presented lesions characteristic of Candida infection. These findings showed efficacy of PDT in treating candidiasis lesions.
Histological sections verified few hyphae in the epithelium and the presence of tissue lesions, including the loss of filiform papillae, hyperparakeratosis, exocytosis, disorganized epithelium and chronic inflammatory infiltrate in the lamina propria. These lesions are all characteristic of the development of experimental candidiasis on rat tongues and have been reported by several authors, including Allen et al. [18], Jorge and colleagues [19] and Junqueira and co-workers [14].
Analysis of the results of Candida quantification in the epithelium revealed no statistical differences between the groups for any of the euthanasia times studied. This most likely occurred because, at these times, the majority of rats no longer showed Candida colonization on the tongue dorsum, and, in those where the microorganism persisted, colonization occurred with few hyphae. According to Samaranayake and Samaranayake [20], during the development of experimental candidiasis, the yeasts and hyphae are eliminated from the organism by the host’s immunological system.
Thus, the most significant results of our work were related to the presence of tissue lesions, characterized by alterations in the epithelial tissue and by inflammatory infiltrate of the lamina propria. Statistically significant differences were observed between the groups only in rats killed 1 day or 5 days after PDT. Rats euthanized immediately after PDT exhibited no differences from the remaining groups. These findings revealed that the rats responded to the effects of PDT within 24 h of its application.
For the euthanasia times of 1 day and 5 days, differences in tissue lesion intensity were observed between the L+P+ and L−P− groups and the L+P− and L−P− groups.
The group that was given PDT (L+P+) exhibited fewer epithelial alterations and a lower chronic inflammatory response than those in the L−P− group, such that only one out of six rats killed after 1 day and three out of six rats killed after 5 days were attributed a score of 0 for epithelial alterations and inflammatory infiltrate. Analysis of these data suggests that the PDT realized in this work showed efficacy in the treatment of experimental candidiasis; however, in the majority of the rats, the infectious process was not completely eliminated. In the search for better results when PDT is used in the treatment of buccal candidiasis, further in vivo work is required and should include more PDT sessions.
The group treated with the laser only (L+P−) presented more intense epithelial alterations and chronic inflammatory response than the remaining groups, showing a statistically significant difference between the L+P− and L−P− groups. Almeida et al. [21] evaluated the effects of PDT using methylene blue on the progression of induced periodontal disease in rats, using histological and radiographic examinations. They verified a diminished evolution of periodontal disease in rats that were subjected to PDT. However, in rats treated with laser only, an intense chronic inflammatory reaction was observed in the conjunctive gingival tissue, which was greater than in the remaining groups (L+P+, L−P+ and L−P−). The authors related these effects to the capacity of low-intensity laser therapy to promote angiogenesis, cell proliferation, accelerated collagen synthesis, inflammation, and tissue repair [21]. Karu [22] contraindicates the isolated use of a laser in infected areas, with no emphasis on the ability of the laser to stimulate the host biologic response and, therefore, the action of the patient’s immunological system against infection.
Analysis of the results of this work suggested that photodynamic therapy could be useful for the treatment of buccal candidiasis. However, further studies are required to establish an ideal protocol for photodynamic therapy and to elucidate the effects of low-intensity laser only in lesions infected by yeasts of the Candida genus.
Conclusion
The rats treated by photodynamic therapy developed more discrete buccal candidiasis lesions than did the remaining groups.
References
Sullivan DJ, Morgan GP, Pinjon E et al (2004) Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res 4:369–376
Wilson M, Mia N (1993) Sensitisation of Candida albicans to killing by low-power laser light. J Oral Pathol Med 22:354–357
Teichert MC, Jones JW, Usacheva MN, Biel MA (2002) Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93:155–160
Donnelly RF, McCarron PA, Tunney MM (2008) Antifungal photodynamic therapy. Microbiol Res 163:1–12
Jori G, Fabris C, Soncin M et al (2006) Photodynamic therapy in the treatment of microbiol infections: basic principles and perspective applications. Lasers Surg Med 38:468–481
Maisch T (2007) Anti-microbial photodynamic therapy: useful in the future? Lasers Med Sci 22:83–91
Konopka K, Goslinski T (2007) Photodynamic therapy in dentistry. J Dent Res 86:694–707
Bouillaguet S, Owen B, Wataha JC, Campo MA, Lange N, Screnzel J (2008) Intracellular reactive oxygen species in monocytes generated by photosensitive chromophores activated with blue light. Dent Mater 24:1070–1076
Chabrier-Roselló Y, Foster TH, Pérez-Nazario N, Mitra S, Haidaris C (2005) Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob Agents Chemother 49:4288–4295
Souza SC, Junqueira JC, Balducci I, Koga-Ito CY, Munin E, Jorge AOC (2006) Photosensitization of different Candida species by low power laser light. J Photochem Photobiol B 83:34–38
Munin E, Giroldo LM, Alves LP, Costa MS (2007) Study of tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J Photochem Photobiol B 88:16–20
Allen CM, Blozis GG, Rosen S, Bright JS (1982) Chronic candidiasis of the rat tongue: a possible model for human median rhomboid glossitis. J Dent Res 61:1287–1291
Reed MF, Scragg MA, Williams DM, Soames JV (1990) In vivo effects of Candida albicans products on rat oral epithelium. J Oral Pathol Med 19:326–329
Junqueira JC, Colombo CED, Martins JS, Koga-Ito CY, Carvalho YR, Jorge AOC (2005) Experimental candidosis and recovery of Candida albicans from the oral cavity of ovariectomized rats. Microbiol Immunol 49:199–207
Bliss JM, Bigelow CE, Foster TH, Haidaris CG (2004) Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother 48:2000–2006
Kömerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M (2003) In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother 47:932–940
Yujra VQ, Scherma AP, Junqueira JC, Jorge AOC, Rocha RF (2006) The role of metronidazole on the establishment and persistence of oral candidosis. Braz J Oral Sci 5:1041–1047
Allen CM, Paulson R, Duncan R (1989) Clinical, histologic and scanning electron microscopic study of the development of chronic candidiasis of the rat tongue. J Oral Pathol Med 18:352–359
Jorge AOC, Totti MAG, Almeida OP, Scully C (1993) Oral candidiasis established in the sialoadenectomized rat. J Oral Pathol Med 22:54–56
Samaranayake YU, Samaranayake LP (2001) Experimental oral candidiasis in animal models. Clin Microbiol Rev 14:398–429
de Almeida JM, Teodoro LH, Bosco AF, Nagata MJH, Oshiiwa M, Garcia VG (2007) Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol 78:566–575
Karu T (1989) Photobiology of low-power laser effects. Health Phys 56:691–704
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil, grant no. 05/55084-8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junqueira, J.C., da Silva Martins, J., Faria, R.L. et al. Photodynamic therapy for the treatment of buccal candidiasis in rats. Lasers Med Sci 24, 877–884 (2009). https://doi.org/10.1007/s10103-009-0673-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0673-4