Abstract
The already known benefits produced by the interaction of coherent light (laser) with biologic tissues determine its use as an adjuvant in the treatment of several complications associated with diabetes. Non-coherent light, such as that emitted by light emitting diodes (LEDs), becomes a promising alternative, because of its low cost and easy handling in these applications. Thirty-six rats were given surgical dorsum lesions. The lesions for the control group did not receive any supporting therapy. The other groups were irradiated only once, 30 min after the establishment of the lesion, with LED (640 nm with 40 nm full bandwidth at half maximum) or laser (660 nm). The histomorphological and histomorphometrical parameters were quantified. The coherent and non-coherent lights produced similar effects during a period of 168 h after the lesions had been made. For the group composed of diabetic animals, 72 h after creation of the lesion, it was observed that the therapy with LEDs had been more efficient than that with the laser in the reduction of the wounds’ diameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is one of the prevalent non-contagious diseases in the world population. DM type 1 is responsible for approximately 10% of primary diabetes. Most of the 80–90% of remaining patients have diabetes type 2. Although the two types of diabetes have different pathogenic mechanisms, the general complications are similar [1]. The histological alterations deriving from hyperglycemia in this disease are present in several tissues, since it is a systemic disorder. These alterations lead to the incidence and progression of other disorders in the vascular system, kidneys, and nervous system, and are manifested in retardation of tissue repair in cutaneous wounds [2, 3]. Several studies have demonstrated that phototherapy improves tissue repair through several mechanisms.
Phototherapy probably increments the production of adenosine triphosphate (ATP) [4], increases the synthesis of protein [5, 6], stimulates the microcirculation by improvement of nutritional contribution, which, associated with an increase in mitotic activity, results in facilitated cellular multiplication and new vessel formation from existing vessels [7–10]. It contributes to the deposition of collagen fibers, in the initial phase of the treatment and also in the late phase, as well as to the reduction in the number of inflammatory cells in the lesion zone in the initial phase of the cicatricial process [11, 12].
With the introduction of light emitting diodes (LEDs) in light therapy, several debates have been generated about differences from and similarities to lasers [13, 14]. Several research studies have verified the efficiency of therapy with LEDs in tissue repair [15, 16], but only a few of them compare LEDs with laser or involve organisms systemically compromised by some abnormality. In this way, phototherapy deserves to be distinguished as an effective therapeutic modality for the minimization of adverse effects caused by diabetes. In addition, the small number of published studies and the lack of conclusive data on the use of optical radiation from LEDs, in light therapy, motivate further investigation in this field.
Our work was an experimental investigation and a comparative study of the biomodulation effects produced by either coherent or non-coherent lights on the initial process of tissue repair in diabetic (type 1) rats. Considering the fact that DM type 1 and type 2 generate similar complications in the organism [1], the results found in our study can be inferred for the treatment of DM types 1 and 2.
Materials and methods
Ethical aspects
This study was submitted for verification by the Research Ethics Committee of the Vale do Paraíba University and obtained approval, under protocol number A058/CEP/2007, following ethical aspects of animal testing according to the principles of the legislation for animal research.
Animals
We used 54 rats (Rattus norvegicus albinus, Rodentia mammalia), Wistar family, male adults, with weights between 180 g and 250 g. The animals were submitted to an adaptation period of 10 days in the vivarium, where they were under an average temperature of 22°C and light and dark cycles of 12 h. They were fed with standard diet, water and rations ad libitum.
Chemical induction of diabetes
Diabetes was induced in 36 animals, chosen randomly, through intravenous administration of 42 mg/kg of monohydrate of alloxan (Sigma Aldrich Inc., St. Louis, MO, USA), diluted with sterile saline solution. The alloxan was injected slowly, in one of the caudal veins.
The animals were starved for 24 h, but they were provided with water ad libitum. At the end of this period they were put in a box, heated by an incandescent lamp at a temperature of approximately 40°C, for 10 minutes, to allow us better visualization of the caudal veins. They were weighed and anesthetized with intra-muscular administration of ketamine hydrochloride (1 g/kg body weight) and xylazine hydrochloride (0.02 g/kg body weight).
Of the 36 animals submitted to diabetes induction, one died during the induction procedures (2.8%) and six rats died during the first 6 days after induction (16.7%). Of the 29 animals left, 11 were removed from the study because they had not acquired the levels of glycemia considered adequate for the characterization of diabetes within pre-established parameters (glycemia less than 180 mg/dl). The 18 animals selected were separated into three experimental groups, each containing three animals.
Glucose determination
For blood collecting and determination of glycemic levels the animals were starved for 12 h, being allowed only water during this period. For all collections, the animals were previously anesthetized. On the first and seventh days we collected the blood by puncturing the venous retro-orbital plexus with a capillary glass tube. The last collection, on the day that the animal was killed, was with a cardiac punch [17]. The collected blood was conditioned in collection tubes containing clot accelerator [silicon dioxide (Si02)] and separator gel. The serum was obtained by centrifugation at 2,400 r.p.m. for 12 min. The immediate glucose dose in the serum was determined by the colorimetric–enzymatic method for glucose oxidase.
Experimental groups and full-thickness wound procedure
The 36 animals selected for the study were separated into six experimental groups, with six animals each, three groups of diabetic rats and three groups of non-diabetic rats, as illustrated in Table 1.
Each animal was given a circumferential lesion, with an area of 0.5 cm2, on the right side of its dorsum, performed with surgical punch 0.8 cm in diameter. For the lesion procedure, the animals were anesthetized and the back pelage was shaved. The animals were then separated into groups (n = 6), as in Table 1.
Treatment parameters and equipment data
For the treatment of groups 2 and 5, LED equipment was used [gallium–aluminum–arsenide (GaAlAs)] with 40 nm bandwidth [full width at half maximum (FWHM)], centered at 640 nm and with 30 mW of optical output power, which was a prototype built for the experiment. For the treatment of groups 3 and 6, laser equipment with continuous wave emission [indium–gallium–aluminum–phosphide (InGaAlP), 660 nm, Endophoton® P30P660-020, KLD Biosistemas, Camanducaia, SP, Brazil was used. For groups 2, 3, 5 and 6 an optical power of 30 mW, distributed in an irradiation area of 0.5 cm2, was used. Therefore, with an irradiation time of 100 s, the applied energy density was 6 J/cm2. The distance between the tip of the irradiation source and the animal’s skin was 1 cm. The animals were irradiated with only one dose, 30 min after the surgical procedure.
The optical output power of the light sources was found before the experiments with the use of a broadband power and energy meter: System 13 PEM 001/J (Melles Griot Photonics Components Group, Carlsbad, CA, USA).
Data acquisition parameters
The wound diameters and crust characteristics were observed for 7 days and photographs of the lesion were taken daily. A 3 megapixel digital capture camera with fixed focus (Moticam® 2300, Motic Group, Xiamen, China) was used, with an acrylic spacer tube coupled to the lens. A reference ruler with a millimetric scale was positioned for dimension measurement.
The lesional diameters on each photograph were analyzed with ImajeJ software (version 1.39u). The evaluation considered the Ferret diameter of the ellipse formed by the wound’s margin lineament.
After the seventh day of experiment the animals were killed and the wounds were removed. The removed tissues were stained with hematoxylin and eosin for histological examination. The photomicrographs obtained from the histological samples were analyzed digitally with the ImageJ software for the quantification of inflammatory cells, blood vessels and fibroblasts.
For histopathological analysis we observed the superficial area of wound tissue and the dermis underneath. Tp study the evolution of wound healing, we analyzed the degree of re-epithelialization (number of cell lines), granulation tissue formation, inflammatory cellular migration, fibroblast proliferation, collagen deposition, and neovascularization [18].
Statistical analysis
We used GraphPad InStat V. 2.05 software to carry out the statistical analysis. The one-way analysis of variance (ANOVA), complemented by the Bonferroni test, was applied to the experimental data. The statistical tests were applied to compare the number of inflammatory cells, vessels and fibroblasts quantified for the LED or laser-treated and control groups, as well as for diabetic and non-diabetic animals.
Results
Clinical and laboratory course of the animals
The glucose concentration (in milligrams per deciliter) and weight of the animals were monitored during the experimental period. Table 2 shows the evolution of the animal weights during the experimental period. The weights of the animals differed in different experimental groups, demonstrating an increase of approximately 9% in non-diabetic animals over a period 14 days (P < 0.01). In the animals with induced diabetes, the mean weight decreased by about 11% over the 14 days of the experiment (P < 0.001).
The values for glycemia are disclosed in Table 3 and allowed us to evaluate the success of the method we used to induce diabetes. The group of diabetic animals had glycemia values significantly higher than those of the non-diabetic animals. The values varied between 70 mg/dl and 90 mg/dl for non-diabetic animals and were above 180 mg/dl for diabetic animals.
Macroscopic evolution of wound repair
The wound diameter was significantly reduced in the groups submitted to phototherapy. The differences were more evident on the diabetic animals. In the non-diabetic group the reduction in wound diameter in the period of 168 h after the surgery was 39.3% for the control group, 50.5% for the group treated with LED and 51.5% for the groups treated with laser. The differences were statistically significant when the treated groups were compared with the non-treated ones (P < 0.05).
For the diabetic animals, the diameter reduction was 30.1% for the control group, 45% for the group treated with LED and 44.5% for the group treated with laser. There was also a statistically significant difference for the light-treated groups in relation to the control group (P < 0.01).
The diabetic group treated with LED, compared with the diabetic control group, showed a statistically significant difference in the reduction of wound diameter in a period of 72 h after surgery.
In a period of 168 h after surgery, the light-treated diabetic animals, in comparison with the non-treated control group, also showed a significant reduction in wound diameter (P < 0.01).
The crust began to form on the wounds around the 48th hour following surgery. At that moment, it was observed that 50% of the non-diabetic animals from the control group already had a crust formed, against 20% of the control group of diabetic animals. In the 72 h subsequent to the establishment of the lesions all the animals showed crust formed on them.
The treated animals showed a more precocious formation of the crust. The crust began to form 48 h after surgery in 66.7% of the non-diabetic, light-treated animals, in 50% of the diabetic animals treated with LED, and in 20% of the diabetic animals treated with laser. The loss of crust began to be observed 120 h after the surgery in the non-diabetic, light-treated animals. In light-treated diabetic animals and diabetic controls, as well as in the non-diabetic control group, the beginning of crust loss was observed later, after 144 h.
Microscopic evolution of wound repair
The microscopic analysis was performed at the end of the experimental period (14th day from the beginning of the experiment). Inflammatory cells, blood vessels and fibroblasts, shown in Table 4, were counted in a delimited area with ImajeJ software. The values reported represent the means of two counts in different dermal region, a shallow one and a deep one. The counting was done on photomicrographs with a magnification of ×200 and covered a cut area of 272 μm2, considered after the calibration of the software imageJ.
For the experimental group of diabetic animals the number of inflammatory cells in the shallow dermis region in the light-treated animals was reduced by approximately 23%, in comparison with the non-irradiated control group. For the deep dermis, the reduction was 19%. For the group of non-diabetic animals, the reduction in the inflammatory cell count, after light-treatment, was 21% and 8% for the shallow and deep dermis, respectively.
The count for inflammatory cells in a diabetic subgroup was always higher than that obtained for the equivalent non-diabetic subgroup, i.e., when we compared the light-treated diabetic subgroup with the light-treated non-diabetic subgroup, as well as when we compared both the diabetic and the non-diabetic controls. When the data for the shallow region of the dermis were compared with the data for the deep portion of the dermis, within equivalent subgroups, the inflammatory cell count was always higher for the shallow region. That held for both the diabetic and non-diabetic experimental groups.
There was a statistically significant difference between the mean numbers of blood vessels in the groups. The numbers of vessels in the diabetic control group, compared with those in the non-diabetic control group, was reduced by approximately 29% in the shallow dermis and by about 35% in the deep dermal region.
It can be seen in Table 4 that the light treatment promoted an increase in the numbers of vessels in both observation sites, i.e., in the shallow and deep dermis. Therefore, it is interesting to note that while the light-treatment promoted similar levels of new vessel formation in the shallow dermis in both diabetic and non-diabetics groups, the same did not hold for the deep dermis. For the deep dermis we observed that, although the light-treatment promoted an effective increase in the numbers of vessels in the treated diabetic group compared with the diabetic control, the counts were always lower than those obtained for the light-treated non-diabetic animals.
When the fibroblast counts (Table 4) for the light-treated groups were compared with those in the respective controls, the statistical tests returned no statistically significant differences, because of the large standard error of the mean (SEM). Despite the small differences, the fibroblast counts were systematically higher in the light-treated groups than in their respective controls. This lack of randomness suggests that there was an actual increase in the numbers of fibroblasts in the light-treated groups, and that held for both observation sites, i.e., shallow and deep regions of the dermis, as well as for both diabetic and non-diabetic groups.
Qualitative microscopic analysis
The non-diabetic animals (control group) showed normal wound healing. Moderate epithelialization and numbers of inflammatory cells were seen in areas of the superficial dermis. The granulation tissue was less intense at the center of the wound and more intense at the edges, with few collagen fibers and few capillaries. In contrast, the treated groups showed complete epithelialization, represented by a thin tier of epithelial tissue, predominance of fibroblasts in the inflammatory infiltrate, and sparse granulation tissue at the center of the lesion, accompanied by a few collagen fibers, and sometimes with adipose tissue underneath the epithelium. In these groups the vascularization was moderate, with congested capillaries.
In the diabetic animals, the control group did not show any epithelialization. Histological specimens with cell content predominantly inflammatory cells, with discrete vascularization and absence of collagen fibers, were obtained. The treated groups presented histological characteristics that indicated acceleration of the repair process. The epithelialization was observed to cover half, or even less, of the wound in most of cases. The inflammatory infiltrate was frequent but less intense than in the diabetic control group. There were proliferating fibroblasts, few collagen fibers and a discrete presence of blood vessels.
The histological characteristics indicated an acceleration of the cicatrization process by the phototherapy, more evident in the treated groups of diabetic animals.
Discussion
This study was intended to compare the action of phototherapy with that of optical radiation produced by a light-emitting diode (non-coherent light) and by laser (coherent light) in diabetic (type 1) and non-diabetic animals. We consider here the existing evidence that diabetes (type 1 or 2) and phototherapy were factors that, respectively, retarded and accelerated the tissue repair process. As an experimental model, we chose the Wistar rat, considering its ease of handling and its well-known characteristics regarding the induction of diabetes.
Alloxan was chosen because experimental diabetes induced by this medicament reproduces, in Wistar rats, the classical signs of human diabetes, such as glycosuria, polyphagia, polydipsia, and hyperglycemia, and also because of its well-known use in the induction of experimental diabetes.
The follow-up of the animals’ clinical course permitted us to affirm that the 18 non-diabetic animals progressed without any significant alteration during the experimental period. The parameters observed, such as weight and food and water ingestion, kept within the expected levels for healthy adult rats. On the other hand, the animals submitted to alloxan administration showed a significant increase in the glycemia, with progressive loss of weight, increase in food and water ingestion, and increase in diuresis. These symptoms are compatible with the establishment of diabetes [15, 19].
The efficacy of phototherapy in the acceleration of cutaneous wound repair is widely known. In spite of several studies in the area, many of them are still contradictory or do not show positive results for phototherapy [20–23]. Controlled studies comparing laser and LED are scarce, and studies involving wound healing in diabetic animals treated with coherent light versus non-coherent light (equal doses) have not been presented until now. It should be highlighted herein that coherent optical radiation produced by laser sources is intrinsically monochromatic and is expected to be more chromophore specific than the broadband light emitted by such non-coherent sources as LEDs. Broadband non-coherent light can simultaneously excite multiple chromophores and, therefore, might trigger multiple biochemical reactions.
The wound healing process is typically quantified clinically by the approximation of tissue and its margins, and most of the studies used the diameter as the parameter for this verification [11, 15]. Within this perspective, in this study, the benefits of phototherapy were evident, because the wound diameters were significantly reduced during a period of 168 h after surgery in all groups treated with LED or laser. This reduction was more evident in the diabetic groups than in the non-diabetic groups, corroborating the data found in prior studies [11, 24, 25].
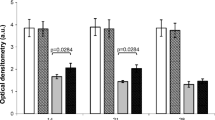
In this study the diabetic group treated with LED demonstrated a significant reduction in wound diameter 72 h after surgery when compared with the diabetic control group and also with the laser-treated diabetic group. The results permit us to affirm that the coherent light and the non-coherent light showed similar effects during the 168 h after surgery Fig. 1.
In the normal repair process, the period of 72 h corresponds to the end of activity of the granulocytes and epithelial cells. In diabetic animals, however, we know that there is an increase in the numbers of inflammatory cells in the lesion, with macrophages congested in relation to the material absorbed [26]. Also, there is evidence that insulin is a pro-inflammatory hormone and, in its absence, all the phases of wound repair can be compromised [27]. The results seen in the LED groups after 72 h of the repair process suggest the existence of biostimulatory effects of this non-coherent radiation. These data corroborate those of authors who affirm that the photochemical effects happen independently of light coherence, because coherence is lost in the first extract of skin, before they produce light absorption by chromophores. There is evidence that both coherent light and non-coherent light are able to stimulate cell proliferation in vitro, to promote the functional activity of exudate leucocytes in cutaneous wounds in rats, stimulating the transition of the inflammatory phase to the proliferative phase and stimulating the activity of the superoxide dismutase enzyme and the formation of nitrogen oxide by phagocytes in the fluid of wounds, decreasing oxidative stress [28, 29]. These results are very important in two principal ways: (1) wound healing is improved with LED therapy, a result which is similar to that obtained with laser therapy; (2) the effects on granulation tissue are very important in both DM type 1 and type 2.
Some anti-inflammatory mechanisms of irradiation with LED in the inhibition of prostaglandin E2 (PGE2) and cyclo-oxygenase (COX) are known [30] and can also explain the action of this kind of irradiation found in our study. Beyond anti-inflammatory effects, phototherapy with LED can increase the synthesis of collagen, having been used for some time in dermatology for rejuvenation treatment [31, 32].
For some authors, angiogenesis is one of the most important histological characteristics of granulation tissue for the process of skin cicatrization [33], since it is evident in several studies conducted with low-power laser therapy [34, 35]. Currently, there is evidence that coherence is not demanded for its establishment [16].
There is evidence that coherent and the non-coherent lights produce similar effects in biologic tissues. In this study this fact could also be observed. After 72 h of the repair process, we observed that the treatment with non-coherent light from an LED source produced enhanced results, when compared with the treatment with coherent laser light. We suggest that this enhanced efficiency may be related to the broad spectrum of the non-coherent light source, instead of coherence or light-polarization issues. The availability of multiple wavelengths may simultaneously excite multiple chromophores and trigger multiple biochemical reactions at the same time, which may not happen with monochromatic laser radiation. It can be observed that most of the previously reported work performed with LED sources worried too much about coherence and forgot about the spectral features of the light source. In addition, it may also happen in many studies that the wavelength of the monochromatic laser radiation did not match the peak response of a target chromophore. In this sense, wavelength matching for optimum chromophore response would be warranted by the broader LED emission spectra. However, further studies are necessary to explain the physiologic mechanisms involved in these effects.
Conclusions
Irradiation with laser InGaAlP (6 J/cm2 at 660 nm) or LED (640 nm with 40 nm full-bandwidth at half maximum) promoted, in a similar way, a slight acceleration of the cutaneous wound healing process when compared with that in an untreated group. This effect was observed in both diabetic and non-diabetic rats, although it was more evident in the diabetic animal groups.
References
Cotran RS, Kumar V, Collins T (2004) Robbins pathologic basis of disease. Saunders
Renvert S (2003) Destructive periodontal disease in relation to diabetes mellitus, cardiovascular diseases, osteoporosis and respiratory diseases. Oral Health Prev Dent 1 (Suppl 1):341–357
Jakus V, Rietbrock N (2004) Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res 53:131–142
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol Chem 27:219–223. doi:10.1016/1011–1344(94)07078–3
Greco M, Guida G, Perlino E, Marra E, Quagliariello E (1989) Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun 163:1428–1434. doi:10.1016/0006–291X(89)91138–8
Passarella S, Ostuni A, Atlante A, Quagliariello E (1988) Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem Biophys Res Commun 156:978–986. doi:10.1016/S0006–291X(88)80940–9
Ihsan FRM (2005) Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg 23:289–294. doi:10.1089/pho.2005.23.289
Schindl A, Heinze G, Schindl M, Pernerstorfer-Schon H, Schindl L (2002) Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res 64:240–246. doi:10.1006/mvre.2002.2429
Schindl A, Schindl M, Schon H, Knobler R, Havelec L, Schindl L (1998) Low-intensity laser irradiation improves skin circulation in patients with diabetic microangiopathy. Diabetes Care 21:580–584. doi:10.2337/diacare.21.4.580
Seppala B, Sorsa T, Ainamo J (1997) Morphometric analysis of cellular and vascular changes in gingival connective tissue in long-term insulin-dependent diabetes. J Periodontol 68:1237–1245
Rabelo SB, Villaverde AB, Nicolau R, Salgado MC, Melo Mda S, Pacheco MT (2006) Comparison between wound healing in induced diabetic and nondiabetic rats after low-level laser therapy. Photomed Laser Surg 24:474–479. doi:10.1089/pho.2006.24.474
Woodruff LD, Bounkeo JM, Brannon WM, Dawes KS, Barham CD, Waddell DL, Enwemeka CS (2004) The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg 22:241–247. doi:10.1089/1549541041438623
Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MTT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M (2003) Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg 21:67–74. doi:10.1089/104454703765035484
Whelan HT, Smits RL, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19:305–314. doi:10.1089/104454701753342758
Al-Watban FA, Andres BL (2006) Polychromatic LED in oval full-thickness wound healing in non-diabetic and diabetic rats. Photomed Laser Surg 24:10–16. doi:10.1089/pho.2006.24.10
Corazza AV, Jorge J, Kurachi C, Bagnato VS (2007) Photobiomodulation on the angiogenesis of skin wounds in rats using different light sources. Photomed Laser Surg 25:102–106. doi:10.1089/pho.2006.2011
Waynforth HB, Flecknell PA (2004) Experimental and surgical technique in the rat. Academy Press
Yu W, Naim JO, Lanzafame RJ (1997) Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med 20:56–63. doi:10.1002/(SICI)1096–9101(1997)20:1<56::AID-LSM9>3.0.CO;2-Y
Maiya GA, Kumar P, Rao L (2005) Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Surg 23:187–190. doi:10.1089/pho.2005.23.187
Allendorf JDF, Bessler M, Huang J, Kayton ML, Laird D, Nowygrod R, Treat MR (1997) Helium-neon laser irradiation at fluences of 1, 2, and 4 J/cm(2) failed to accelerate wound healing as assessed by both wound contracture rate and tensile strength. Lasers Surg Med 20:340–345. doi:10.1002/(SICI)1096–9101(1997)20:3<340::AID-LSM13>3.0.CO;2-H
Petersen SL, Botes C, Olivier A, Guthrie AJ (1999) The effect of low level laser therapy (LLLT) on wound healing in horses. Equine Vet J 31:228–231
Schlager A, Kronberger P, Petschke F, Ulmer H (2000) Low-power laser light in the healing of burns: a comparison between two different wavelengths (635 nm and 690 nm) and a placebo group. Lasers Surg Med 27:39–42. doi:10.1002/1096–9101(2000)27:1<39::AID-LSM5>3.0.CO;2–4
Walker MD, Rumpf S, Baxter GD, Hirst DG, Lowe AS (2000) Effect of low-intensity laser irradiation (660 nm) on a radiation-impaired wound-healing model in murine skin. Lasers Surg Med 26:41–47. doi:10.1002/(SICI)1096–9101(2000)26:1<41::AID-LSM7>3.0.CO;2-M
Pessoa ES, Melhado RM, Theodoro LH, Garcia VG (2004) A histologic assessment of the influence of low-intensity laser therapy on wound healing in steroid-treated animals. Photomed Laser Surg 22:199–204. doi:10.1089/1549541041438533
Reddy GK (2003) Comparison of the photostimulatory effects of visible He-Ne and infrared Ga-As lasers on healing impaired diabetic rat wounds. Lasers Surg Med 33:344–351. doi:10.1002/lsm.10227
Golub LM, Schneir M, Ramamurthy NS (1978) Enhanced collagenase activity in diabetic rat gingiva: in vitro and in vivo evidence. J Dent Res 57:520–525
Leme JG (1981) Regulatory mechanisms in inflammation: new aspects of autopharmacology. Gen Pharmacol 12:15–24. doi:10.1016/0306–3623(81)90022–7
Klebanov GI, Shuraeva NY, Chichuk TV, Osipov AN, Vladimirov YA (2006) Comparison of the effects of laser and light-emitting diodes on lipid peroxidation in rat wound exudate. Biofizika 51:332–339
Klebanov GI, Shuraeva NY, Chichuk TV, Osipov AN, Vladimirov YA (2006) A comparison of the effects of laser and light-emitting diodes on superoxide dismutase activity and nitric oxide production in rat wound fluid. Biofizika 51:116–122
Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H (2007) The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med 39:614–621. doi:10.1002/lsm.20533
DeLand MM, Weiss RA, McDaniel DH, Geronemus RG (2007) Treatment of radiation-induced dermatitis with light-emitting diode (LED) photomodulation. Lasers Surg Med 39:164–168. doi:10.1002/lsm.20455
Weiss RA, McDaniel DH, Geronemus RG, Weiss MA, Beasley KL, Munavalli GM, Bellew SG (2005) Clinical experience with light-emitting diode (LED) photomodulation. Dermatol Surg 31:1199–1205
Biondo-Simoes Mde L, Ioshii SO, Borsato KS, Zimmermann E (2005) The healing process influenced by hypothyroidism and by elderly. Study of abdominal wall healing in rats. Acta Cir Bras 20 (Suppl 1):211–219
Barkovskii VS (1983) Effect of laser radiation on the process of tissue vascularization after damage. Arkh Patol 45:72–76
Del’tsova EI, Neiman AM, Shiian AO (1983) Effect of helium-neon laser rays on the vascular and nerve tissue structures of the small intestine in the cat. Nauchnye Doki Vyss Shkoly Biol Nauki (12):47–50
Acknowledgments
This work was partially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), through grant number 2001/12754-2. The authors would like to thank Celso Erasmo, Laércio Erasmo and Turíbio Carlos Barbosa for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dall Agnol, M.A., Nicolau, R.A., de Lima, C.J. et al. Comparative analysis of coherent light action (laser) versus non-coherent light (light-emitting diode) for tissue repair in diabetic rats. Lasers Med Sci 24, 909–916 (2009). https://doi.org/10.1007/s10103-009-0648-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-009-0648-5