Abstract
A recent trend for converting a hydrophilic lignocellulosic material into oleophilic adsorbent could be achieved by using cationic surfactants. In the present work, the surface of sugarcane bagasse was modified by cationic surfactant cetyltrimethylammonium bromide, whereas sugarcane bagasse acquired hydrophobic properties. Further increase in hydrophobicity of sugarcane bagasse could be obtained by blending surfactant modified sugarcane bagasse with polystyrene waste. Sugarcane bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse were characterized by different physical and chemical techniques. Remarkable changes in the structure of sugarcane bagasse as a result of the different treatment processes could be evidenced by FT-IR, SEM and XRD measurements. Moreover, elemental analysis, specific surface area as well as water absorption capacity results confirmed successful modification of sugarcane bagasse by both cetyltrimethylammonium bromide and polystyrene waste. Also, water absorption capacity experiments indicated that hydrophobic properties of the different samples increased in the order: polystyrene waste/surfactant modified bagasse > surfactant modified bagasse > sugarcane bagasse. The different samples were evaluated for removal of emulsified food oil from aqueous solutions. The effect of various parameters, e.g., blend constituents weight ratio, adsorbent dose, initial oil concentration, pH and contact time upon oil removal efficiency, was investigated. Isothermal studies revealed that oil adsorption fitted Freundlich model and thermodynamics studies showed that oil adsorption is spontaneous, random and exothermic.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Effluents of several industries, such as metal and food processing, petroleum refiners, textiles and paints contain oil in the form of oil-in-water emulsion. Such emulsion is formed of a mixture of poorly degradable light and heavy hydrocarbons (Dumore and Mukhopadhyay 2012), water and emulsifier. The emulsifier can be a detergent or food additives. Oil emulsion is toxic and poses harmful effects on aquatic life and human health, even at low oil concentration, it prevents access of sunlight and oxygen to aquatic organisms by forming a stable layer on the water surface (Zhou et al. 2008).
Several chemical, physical and biological techniques have been developed and investigated to remove oil emulsions in wastewater, such as skimming, gravity setting, filtration, flocculation, electrocoagulation, membrane techniques (Yang et al. 2016), flotation and chemical coagulation (Sangal et al. 2013). However, most of these methods are expensive and time consuming; therefore, many research activities were directed to more effective procedures for oil cleanup, such as adsorption.
Adsorption process has drawn increasing attention as an effective route for remediation of wastewater contaminated with organic and inorganic pollutants. Adsorption process is highly recommended and preferred due to its low cost, simplicity, efficiency and ease of rapid application (Sangal et al. 2013). In the open literature, several adsorbents have been cited and explored for the removal of oil from oil-in-water emulsions, such as activated carbon, biopolymers, organoclay, sawdust, vermiculite, walnut shells and resins (Zhang et al. 2017a, b). In this context, preference should be afforded to cheap and renewable resources. For instance, the past few decades have witnessed the use of agricultural wastes-based adsorbents as efficient candidates for the removal of oil contaminants from aquatic ecosystems. Biodegradable lignocellulosic fibers and natural fibers such as olive wastes, cotton fibers, sago bark (Wahi et al. 2014), barley straw (Ibrahim et al. 2010), rice husk (Ali et al. 2012), bagasse (Said et al. 2009), kenaf (Kundu and Mishra 2013) and corn husk (Pachathu et al. 2016) have been reported as simple and effective materials for oily water treatment.
However, direct application of untreated agricultural wastes results in insufficient adsorption capacity due to the hydrophilic properties caused by the presence of large number of hydroxyl groups. Hence, surface modification of these materials is essential to improve their properties (Ibrahim et al. 2010).
Early trials on surface modification of natural fibers by esterification led to increased hydrophobicity of the fibers and enhanced oil sorption capacity (Wang et al. 2013). Oleic acid modified sawdust, acetylated rice husk, kapok fibers, fatty acid modified bagasse and oleic acid banana trunk fibers are some examples of very effective candidates for oil cleanup operations (Wang et al. 2013). These bioresources could, therefore, be used as substituents for nonbiodegradable oil sorption materials.
Sugarcane bagasse is one of the best lignocellulosic waste materials available in large quantities and low cost. Bagasse is usually used as fuel for sugar mills, for production of paper and pulp (Wikipedia 2015), and also as a good adsorbent for oils and dyes (Aly et al. 2018). Chemical modification of the surface of bagasse is also essential and this could be performed either by esterification of its OH groups using carboxylic acids anhydride (Cavdar et al. 2014) or by graft copolymerization reactions (Fu et al. 2012). Both routes functionalize the surface of bagasse and turn it hydrophobic.
Some recent studies introduced the thermally stable, nonvolatile and recyclable ionic liquids pretreatment step to dissolve lignocellulosic materials for subsequent homogeneous modification processes (Hallett and Welton 2011). Homogeneous acylation and carbonylation reactions in ionic liquids resulted in highly substituted lignocellulosic esters compared to those prepared in heterogeneous conditions (Xie et al. 2007). Also, Chen et al. (2016) reported on homogeneous free radical initiated graft copolymerization of bagasse using hydrophobic acrylate monomers. The results showed that machine oil, cooking oil and diesel oil adsorption capacities of bagasse were remarkably improved due to the presence of acrylate monomers.
Another more recent and promising trend for surface modification of lignocellulosic materials is the use of surfactants. Surfactants are characterized by low cost, biodegradability as well as surface active properties. Surfactants are organic compounds that contain a long hydrophobic tail and positive- or negative charged head. Cationic surfactants carry positive charge on the head and have drawn increasing attention during the last decade as effective surface modifiers for lingo-cellulosic materials. Octadecyltrimethylammonium bromide (C18, OTAB), cetyltrimethylammonium bromide or chloride (C16, CTAB or CTAC), cetylpyridinium bromide or chloride (C16, CPB or CPC), tetradecyltrimethylammonium bromide (C14, TTAB), dodecylpyridinium chloride (C12, DPC) are some examples of widely used cationic surfactants (Marković-Nikolić et al. 2017).
There are few articles that deal with the use of surfactant modified lignocellulosic materials as oil adsorbents. Ibrahim et al. (2009) and Augusta and Kalaichelvi (2016) reported about CPC modified barley straw as sorbent for standard mineral and canola oils in batch adsorption system. Oil adsorption on the surface of the straw was found rapid (equilibrium was reached within 40 min) and high at low adsorbent dosage and small particle size, pH 6.0–8.0 and temperature 20–40 °C. Moreover, desorption was very low indicating strong ion exchange bonding between oil and adsorbent.
Augusta and Kalaichelvi (2016) reported about the use of CPC modified bagasse and corn husk as potential bioadsorbents for emulsified engine oil removal in packed bed column. Surface modification of the two wastes was carried out under normal rotary shaker and advanced environments, such as microwave and ultrasound medium. The modified wastes via microwave and ultrasound exhibited higher adsorption capability than unmodified and rotary shaked modified adsorbents.
Satirawaty et al. (2018) used CTAB modified sago hampas to remove palm-based cooking oil from wastewater. The authors confirmed the existence of additional functional groups on the surface of the modified biomass which possessed higher porosity than unmodified sago. Moreover, optimum oil adsorption was reached after 45 min at pH 2.0 and 0.2 g adsorbent dosage.
In their report, Markovic-Nikolic et al. (2017) focused on how to control the degree of hydrophobicity of the resulted adsorbent. The authors used hexadecyltrimethyl ammonium chloride (HTAC), the inexpensive and water soluble surfactant, stable at acidic and basic pH ranges with strong cationic activity, to modify bottle ground shell (a solid agricultural residue growing in Serbia). The modified biomass enhanced phosphate and nitrate anions sorption.
Another approach to turn bagasse hydrophobic has been carried out by Pan et al. (2016). The surface of bagasse was modified by using a cationic nanosized latex with core–shell structure. The latex was prepared by using CTAC and cationic initiator with appropriate hydrophobicity and film formation capability after adsorption on bagasse. The latex film improved the compatibility between the hydrophilic bagasse and a hydrophobic material for various applications.
Polystyrene waste is low cost, nonbiodegradable material that cannot be easily collected (Chauhan et al. 2008), so its disposal by landfilling or incineration is highly harmful to human health and environment due to the generation of toxic gases such as CO2 and CO (Shah et al. 2014). For instance, converting polystyrene waste into useful material is an avenue to overcome these problems (Hearon et al. 2014). In general, polystyrene waste is chemically stable, characterized by superoleophilicity and high hydrophobicity (Zhang et al. 2015). Researchers have focused on the use of surfactants modified polystyrene (Zhou et al. 2010) and polystyrene-based composites as effective emulsified oil adsorbents. Cetyltrimethyl ammonium bromide/modified polystyrene, resin/granular activated carbon (Wang et al. 2013), porous polystyrene/zeolite (Alayande et al. 2016), polystyrene/polytetrafluoroethylene coated filter paper (Du et al. 2014), acetic anhydride-treated pomelo, peel/polystyrene blends (Chai et al. 2015) are some reported examples.
However, oil adsorbents based on blending unmodified polystyrene waste with surfactant modified bagasse is not satisfactorily investigated. Therefore, the present work aims to convert pristine polystyrene and bagasse wastes into value added material. Modification of bagasse was carried out by using the cationic surfactant (CTAB) followed by blending with polystyrene waste. Surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend were characterized and evaluated as adsorbents for emulsified oil. Effect of different experimental parameters on oil removal capacity and efficiency was investigated. Isothermal models, thermodynamics studies and reusability of the blend were also performed.
Materials and experimental methods
Materials
Depithed sugarcane bagasse waste was obtained from local pulp and paper factory, Quena, Egypt. Bagasse was washed carefully with distilled water to remove dust and then dried at 60 °C. Cetyltrimethylammonium bromide (CTAB, C16H33NCH3Br), a product of Fluka, was used as a cationic surfactant. Expanded polystyrene foam waste in the form of packaging material was used. Food oil was purchased from local market, sodium lauryl sulfate emulsifier (SLS) and sodium hydroxide were from El-Nasr Company for Chemicals, Egypt. All other used solvents and reagents were of analytical grade.
Preparation of surfactant modified bagasse
Bagasse was treated with the cationic surfactant CTAB as follows: A known amount of bagasse was immersed in 0.05 mol/L sodium hydroxide solution for 30 min under magnetic stirring then filtered and washed well with distilled water. NaOH-treated bagasse was immersed in 2.5 mmol/L CTAB solution and magnetically stirred for 24 h at 60 °C. CTAB-treated bagasse was separated and washed carefully several times with distilled water to remove unreacted surfactant. At the end, the product was dried overnight in an oven at 60 °C and kept in sealed bottle.
Preparation of polystyrene waste/surfactant modified bagasse blend
1 g of polystyrene waste was washed with distilled water to remove dust, dried in an oven at 60 °C for 2 h and then dissolved in 50 mL acetone. 1 g of surfactant modified bagasse was added to polystyrene waste solution, and the mixture was stirred magnetically for 1 h. After, the mixture was casted in Petri dish and allowed to dry overnight. The blend was washed with distilled water and dried in an oven at 60 °C for 3 h. Different polystyrene wastes: surfactant modified bagasse blend weight ratios (0.25, 0.5, 1 and 2) were prepared.
Preparation and characterization of emulsified oil wastewater
Food oil–water emulsion stock solution was prepared as reported by Alther (2001). A known weight of food oil was mixed with definite weight of the emulsifier (SLS) in one liter of distilled water. The mixture was homogenized by using high speed blender for 10-30 min until milky solution was obtained. This stock oil emulsion was diluted with distilled water to prepare different oil concentrations.
pH of food oil–water emulsion was measured by using a pH meter of model HANNA HI98130. The total dissolved solids (TDS) measurements were performed by gravimetric method. The turbidity of food oil–water emulsion was recorded by turbidimeter of model HACH 2100P. Table 1 represents the values of pH, turbidity and total dissolved solids (TDS) for food oil–water emulsion.
Characterization of adsorbents
Fourier transform infrared spectroscopy (FT-IR)
The changes in the vibrational frequency and intensity of native bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse were investigated by FT-IR spectroscopy. Known weight of the samples and KBr were pressed into disks and measured on JASCO FT-IR-6100 spectrometer. The spectra were recorded in the frequency range 4000–400 cm−1 at room temperature with resolution 4 cm−1.
Scanning electron microscopy (SEM)
The surface morphology of the raw and modified sorbents was detected by using JEOL JXA-840A electron probe microanalyzer. The acceleration of the electron beam was 10 kV.
Powder X-ray diffraction (XRD)
X-ray diffraction patterns of bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse were recorded using BRUKUR D8 ADVANCE diffractometer in the range of 2Ө = 10–80°. The instrument operates at 40 kV and 40 mA with Cu Kα monochromator (λ = 1.5405 A°) radiation.
Specific surface area, average pore diameter and pore volume
Brunauer–Emmett–Teller (BET) method (Brunauer et al. 1938) and Barrett–Joyner–Halenda (BJH) model (Barrett et al. 1951) were employed for estimation of specific surface area, average pore diameter and pore volume, respectively, of bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend instrument of the type Autosorb-Quantachromium analyzer (NOVA-2000). USA was used to perform these measurements by applying N2 adsorption/desorption at 77 K. Before measurements, degasification of the samples was carried out by heating at 60 °C.
Elemental analysis
Carbon and nitrogen percent of bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend were detected by using an Elementar, Vario Macro CHNS analyzer.
Point of zero charge (pHPZC)
The point of zero charge pH(PZC) for surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend was estimated by the solid addition method (Cueva-Orjuelaa et al. 2017). To a series of 100 ml conical flasks, 50 ml of 0.1 N KNO3 solution was transferred. The pH values of the solutions were adjusted from 2.0 to 10.0 by adding either 0.1 N HCl or NaOH and denoted as pHi. 1.0 g of sample was added to each flask then the flasks were capped, the suspensions were mixed thoroughly and allowed to equilibrate for 48 h. At equilibrium, the final pH (pHf) values of the supernatant liquids were recorded. (pHi–pHf) values were plotted against pHi, and pH(PZC) was determined from the point of intersection of the resulting curve at which (pHi–pHf) is zero.
Water absorption capacity (WAC)
Water absorption capacity (WAC) values for bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend were recorded by placing each adsorbent in a beaker containing 100 mL distilled water for a specific time period (10–100 min). The wet adsorbent was drained on a filter paper, and the water absorption capacity (WAC) of each sample was calculated as follows (Hafshejani et al. 2016):
where W1 is the weight of the dry sample (g) and W2 is the weight of the wet sample (g).
Oil sorption test
Different batch adsorption experiments were performed by mixing a known weight of each sorbent with 100 mL of emulsified oil wastewater with different initial oil concentrations, the mixture was stirred at 25 °C until equilibrium reached. After certain time, the sorbent was separated, and the remaining concentration of emulsified oil-in-water was measured by FT-IR spectrophotometer of the type A-4600 according to IS 3025 (part 39) (Bismarck et al. 2002) and IS 10,500 methods (Ibrahim et al. 2009). Typically, spectra were acquired over the range 3200-2700 cm−1 at 4 cm−1 resolution with ~ 1 min acquisition time. Blank experiment with no adsorbent was also carried out.
The residual emulsified food oil/water mixture was acidified with hydrochloric acid to pH 2.0. The residual oil was extracted by adding a known amount of CCl4 three times under stirring for adequate time period. A separating funnel was used to separate oily phase from aqueous phase, the oily phase was filtered through filter paper containing 1 g anhydrous sodium sulfate, and the residual food oil concentration was deduced from calibration curve, i.e., plot of series of definite concentrations against sum of peaks hight values (for aromatic CH at 3100 cm−1, aliphatic CH3 at 2930 cm−1 and aliphatic CH2 at 2860 cm−1). Finally, oil concentration in mg/dm3 was determined from Eq.[2].
where C is the concentration of food oil which can be calculated from the calibration curve.
The oil adsorption capacity (Q in g/g) and removal efficiency for surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend were calculated as follows.
where Ci and Cf are initial and final oil concentrations in g/l at time t, respectively, V is the volume of emulsified oil solution in liter (l) and m is the weight of adsorbent (g).
The effect of different parameters, such as polystyrene waste: surfactant modified bagasse blend weight ratios, initial oil concentration, adsorbent dose, initial pH, temperature as well as contact time was investigated. When a parameter value was changed, all other parameters were kept constant.
Isothermal studies
A batch isothermal study was carried out by mixing different adsorbent dosages in the range (0.5–3 g) with 100 mL of emulsified oil wastewater where the other parameters were kept constant. At equilibrium, the concentration of oil was measured, and the data were analyzed for Langmuir and Freundlich isotherms. All experiments were carried out in triplicate, the mean value was recorded and the best fitting model was reported.
Reusability studies
In order to reuse the oil sorbent several times, desorption of oil from oil saturated samples was carried out. The oil loaded adsorbent was immersed in a beaker containing n-hexane and stirred for 15 min to extract adsorbed oil, afterward, the sorbent was dried in an oven at 60 °C for 3 h. The sorbent was used again for oil sorption. This was repeated for six cycles, and after each cycle oil removal efficiency was recorded.
Results and discussions
CTAB is a cationic surfactant, composed of two parts, nonpolar, hydrophobic alkyl chain tail and positively charged head. From our point of view, the mode of attachment of CTAB to bagasse can be suggested as follows: The alkyl chains may interact with the hydrophoic moieties on the surface of bagasse through hydrophobic-hydrophobic bonding, whereas the positive head (in the form of monolayer) is directed toward the bulk solution (Chung et al. 2011; Chiparus, 2004). Another route is the interaction of the cationic heads (in the form of monolayers) with the negative moieties of bagasse through ion exchange and electrostatic attraction and in this case the nonpolar chain is pointing to the bulk solution, forming a monolayer. A surfactant bilayer or possibly multiple layered forms can also be built up when the alkyl tail of the monolayer is attached to another or many alkyl chains of CTAB via hydrophobic–hydrophobic interaction. Figure 1a,b illustrates the suggested interaction routes. It is supposed that, the obtained results can determine the mode of interaction between CTAB and bagasse. However, a specific concentration of CTAB required to avoid bi- or multilayer formation is not studied yet but may be considered in another work.
Characterization
Point of zero charge (pHPZC)
Point of zero charge (pHPZC) is defined as the pH at which the surface net charge of adsorbent is zero, and the anion and cation exchange capacities are equal (Song et al. 2011). To determine pHPZC for surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend, the difference between initial and final pH (pHi–pHf) is plotted against pH. pHPZC is the pH value at which pHi-pHf is zero. Figure 1c,d displays pHPZC for surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend, respectively. From this figure, pHPZC is about 5.1 and 5.6 for surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend, respectively. At pH lower than pHPZC, the surface of both adsorbents is positively charged due to adsorption of H+ ions, whereas pH values higher than pHPZC indicated negatively charged surface of adsorbents due to desorption of H+ ions.
Elemental analysis, specific surface area, average pore diameter and pore volume
Results of elemental analysis for bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse blend are shown in Table 2. As expected, modification of bagasse with CTAB led to considerable increase in the percentages of the three elements and this confirms the attachment of the quaternary ammonium groups of the surfactant on bagasse surface. Furthermore, blending of the surfactant modified bagasse with polystyrene waste showed further increase in both C % and H %, whereas N % was constant.
In the sum, the surfactant alkyl group and polystyrene layer in modified bagasse have significant impact in the improvement coalescing process between oil droplets and adsorbent, the oil removal process occurred by hydrophobic bonding between hydrocarbon molecules in bulk solution and hydrophobic sites on the adsorbent surface (Gupta et al. 1999).
The values of the measured specific surface area, average pore diameter and pore volume are also given in Table 2. The surface area of bagasse is 0.84 m2/g, it is clear that modification of bagasse with CTAB led to reduction of the surface area, average pore diameter and volume. This could be attributed to deep penetration of the surfactant molecules into the pores of bagasse causing shrinkage of the surface. As reported elsewhere (Ibrahim et al. 2009), surfactant modification of barley straw also caused decrease in the specific surface area from 143.5 to 63.2 m2/g. On the other hand, the specific surface area of the blend exhibited considerable increase but decreased pore diameter and volume. Since polystyrene molecules are built up of C and H only, the constant N % together with increasing surface area, decreasing pore diameter and volume in the blend may suggest the mode of attachment of the surfactant with bagasse, preferrably monolayer form, in which the heads of the surfactant interact with OH groups of bagasse. If there were possibility for existence of bilayers or multilayers in the modified bagasse, polystyrene molecules may have caused reorientation of the surfactant molecules to a monolayer pattern. It can also be said that the enormous increase in surface area of the blend can be attributed to full coverage or coating of surfactant modified bagasse with polystyrene.
Water absorption capacity (WAC)
Figure 1e illustrates the effect of increasing soaking time from 10 to 100 min on the WAC values for bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse, respectively. It is obvious that the hydrophilic bagasse has high affinity to water due to the presence of large number of –OH groups which form hydrogen bonding with water molecules (Chung et al. 2011). Furthermore, the voids in the structure of bagasse enable diffusion of water (Chiparus, 2004). It is also noticed that equilibrium was reached after 80 min. On the other hand, modification of bagasse led to considerable reduction of WAC values and this is a result of the interaction of the positive heads of the surfactant with the –OH groups of bagasse. However, the increasing WAC values by time from 0.77 to 2.3 g/g may indicate the presence of some free –OH groups on the surface of bagasse. Moreover, blending surfactant modified bagasse with polystyrene waste led to pronounced reduction of WAC to 0.35 g/g, which means that polystyrene waste increased the hydrophobicity of the blend, showing remarkable oleophilic characteristics.
On the basis of the obtained results, it can be concluded that WAC measurement is a useful tool to differentiate between hydrophilic and hydrophobic substances. The modification step turned bagasse hydrophobic due to the attachment of the quaternary ammonium group of CTAB to hydroxyl group of bagasse and in this case the nonpolar hydrophobic tail is directed toward water. Interaction of CTAB hydrophobic alkyl chains with polystyrene layer imparts the adsorbent much more hydrophobic properties.
FT-IR spectra
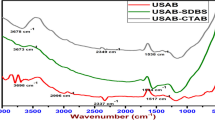
IR spectra of bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse are given in Fig. 2a–c. The main features of the spectrum of bagasse reflect the presence of lignin, hemicelluloses and cellulose. It is to be noted that the assignments of the different bands are collected from different articles (Abdelwahab and Shukry, 2015; Kanwal et al. 2019). The assignments of bagasse (Fig. 2a) can be summarized as follows:
FT-IR spectra of (a) bagasse, (b) surfactant modified bagasse and (c) polystyrene waste/surfactant modified bagasse, SEM micrographs of (d) bagasse, (e) magnified bagasse, (f) surfactant modified bagasse, (g) magnified surfactant modified bagasse, (h) polystyrene waste/surfactant modified bagasse and (i) magnified polystyrene waste/surfactant modified bagasse and XRD spectra of (j) bagasse, (k) surfactant modified bagasse and (l) polystyrene waste/surfactant modified bagasse
The bands appearing at 3430 cm−1 and 2922 cm−1 are assigned to –OH groups, C–H, CH2 and CH3 in lignin, hemicelluloses and cellulose, respectively. Two absorption bands characteristics for C–Ph and C = C in lignin are detected at 1604 and 1633 cm−1, whereas the band at 1735 cm−1 represents acetyl group in hemicellulose. The bands at 1051 and 1165 cm−1 represent primary and secondary –OH groups. The band at 840 cm−1 is the result of glycosidic linkage. In the spectrum of surfactant modified bagasse (Fig. 2b), some changes in bands frequency and intensity are observed confirming the attachment of the surfactant to bagasse. For example, the band at ~ 3400 cm−1 became weaker due to the interaction between –OH groups of bagasse and the quaternary ammonium groups of the surfactant. On the other hand, the band at 2922 cm−1 is shifted to 2968 cm−1, became sharper and stronger, also a new peak at 1580 cm−1 appeared. These changes in the spectrum are due to the CH3 and CH2 of the surfactant. Additionally, the new peak at 900 cm−1 is due to the C–N of surfactant. All these changes give evidence of successful modification of bagasse. Figure 2c illustrates the spectrum of the blend, it has been observed that the intensity of almost all peaks assigned to the surfactant decreased, and a band at 730 cm−1 is recognized, which can be referred to the benzene ring in polystyrene.
Scanning electron microscopy (SEM)
SEM images with two magnifications for bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse are represented in Fig. 2d–i. The surface morphology of bagasse (Fig. 2d) and bagasse with 1500 magnification (Fig. 2e) shows smooth bundles from arranged fibers, while the morphological characteristics of surfactant modified bagasse (Fig. 2f) and its 1500 magnified image (Fig. 2g) indicate that the surface of the bundles turned rough. In case of polystyrene waste/surfactant modified bagasse sample before (Fig. 2h) and after 1500 magnification (Fig. 2i), only thin smooth layers of polystyrene waste adhering to each other and to the surface of surfactant modified bagasse were recognized. These images together with FTIR spectra and WAC results consolidate the finding that there is complete coating of surfactant modified bagasse with polystyrene.
X-ray diffraction (XRD)
Figure 2j–l shows diffractograms of bagasse, surfactant modified bagasse and polystyrene waste/surfactant modified bagasse. As can be seen, the diffractogram of bagasse exhibits typical crystalline cellulose diffraction peaks located at 2θ = 16.5° and 22.0° which are due to the intra- and intermolecular hydrogen bonding (Nazir et al. 2013). There are also some amorphous regions in this diffractogram owing to the presence of hemicelluloses and lignin (Zainuddina et al. 2017). Upon modification of bagasse, reduction in crystallinity peak at 2θ = 22.0° was observed with almost no other changes (Fig. 2h). This was also reported by Kargarzadeh et al. (2012). Furthermore, coating surfactant modified bagasse with polystyrene led to disappearance of the two crystallinity peaks and the structure turned amorphous displaying a new amorphous peak for polystyrene at 2θ = 19.0° (Fig. 2l). This diffractogram confirms good adhesion of polystyrene to the surfactant modified bagasse.
Effect of experimental parameters on oil removal capacity and efficiency
Effect of polystyrene waste: surfactant modified bagasse weight ratios
To investigate the effect of changing polystyrene waste:surfactant modified bagasse weight ratio in the blend on oil removal capacity and efficiency, 0.5 g of the blend was added to 100 mL of emulsified food oil with stirring for 2 h at 25 °C, whereas initial oil concentration was 2 g/l at pH 5.0 (ambient pH). Figure 3a,b shows the effect of different polystyrene wastes: surfactant modified bagasse weight ratios (0.25, 0.5, 1 and 2) on oil adsorption capacity and oil removal efficiency, respectively. It was observed that as the polystyrene waste:surfactant modified bagasse ratio increased from 0.25 to 1, the oil adsorption capacity of the blend increased from 6.8 to 11.8 g/g and oil removal efficiency also increased from 66.8 to 80.6%. Further increase in polystyrene waste: surfactant modified bagasse weight ratio to 2, no change in both parameters occured. Hence, it can be concluded that the optimum ratio of participation in the blend is 1.
Effect of initial oil concentration and contact time
To study the effect of initial emulsified oil concentration on oil adsorption capacity of the blend (at weight ratio 1), different oil concentrations (0.5, 1, 2 and 3 g/l) were examined and the results are given in Fig. 4a. It was observed that the adsorption capacity increased gradually from 6.34, 9.55 to 11.8 g/g when using initial oil concentrations 0.5, 1 and 2 g/l, respectively. Further increase in oil concentration to 3 g/l led to decreasing the oil adsorption capacity to 8.32 g/g. The gradual increase in oil adsorption capacity with increasing oil concentration till 2 g/l is suggested to be the result of increasing collision between oil droplets and the surface of adsorbent leading to increased coalescence probability (Zhou et al. 2009), while at higher oil concentration (3 g/l) oil droplets tend to colloid with each other rather than colloid with the surface of adsorbent. Hence, the coalescence between adsorbent surface and oil droplets will be delayed (Li and Gu, 2005).
Figure 4b shows the effect of increasing contact time on oil removal efficiency. It is clear that the removal efficiency increased by contact time regardless of the experimented oil concentration. The oil removal process from water as a function of time can be divided into two stages, the first is the primary rapid stage during which most of the oil amount can be adsorbed and the most available sites on the blend are occupied. The second stage is slow adsorption stage and it occurs before equilibrium. At lower initial oil concentrations (0.5 and 1 g/l), the equilibrium was reached after 30 and 40 min, respectively, while equilibrium was reached after 50 and 60 min for initial oil concentrations 2 and 3 g/l, respectively. The maximum removal efficiency values at equilibrium referred to initial oil concentrations 0.5, 1, 2 and 3 g/l were 91.4%, 85%, 80.6% and 69.3%, respectively, which means that the maximum removal efficiency at equilibrium clearly decreased by increasing oil concentration.
Effect of initial pH
pH has a great impact on oil removal process due to its role on the charge of surface binding sites and emulsified oil stability (Farah et al. 2007). The effect of varying pH on oil adsorption capacity was investigated, and the results are represented in Fig. 4c. The experiments were carried out at pH range 2.0 to 10.0 at polystyrene waste: surfactant modified bagasse blend ratio, 1, initial emulsified oil concentration, 0.5 g/l and contact time, 30 min (optimum conditions).
As seen from Fig. 4c, increasing the pH from 2.0 to 4.0 resulted in slight increase in oil adsorption capacity from 2.3 to 3.5 g/g. Further increase in pH from 4.0 to 5.0 caused noticeable increase in oil adsorption capacity to reach 11.8 g/g, then a slight increase was observed from pH 5.0 to 8.0. However, raising the pH to 10.0 led to remarkable decrease in oil adsorption capacity.
Demirbas and Nas (2009) suggested that at lower pH values (strong acid medium), desorption of the surfactant from the polymer may take place as a result of repulsion between the generated positive charge on the surface of the polymer and the positive segments of the surfactant leading to reduction of hydrophobicity.
Considering our results, it is clear that the optimum oil adsorption capacity and hydrophobicity could be reached at pH values (5.0 to 8.0), while the basic medium (9.0 to 10.0) caused a slight negative impact on these parameters. At basic medium, reduction of positively charged sites on the adsorbent surface was returned to the presence of hydroxyl ions, also at basic medium, the solubility of emulsified oil occurs and the oil particles turned negatively charged. Consequently, electrostatic repulsion between adsorbent and oil particles led to reduction in oil adsorption capacity (Zhang et al. 2017a, b).
Adsorbent dose and isothermal modeling studies
The effect of adsorbent dose on adsorption capacity and removal efficiency for the studied sorbents was investigated at adsorbent dose ranging from 0.5 to 3 g/l, and the results are illustrated in Fig. 5a. Oil adsorption capacity as well as oil removal efficiency increased from 13.5 to 22.3 g/g and from 94.3 to 98.7%, respectively. Upon increasing sorbent dose from 0.5 to 2 g/l. This can be due to large number of available hydrophobic sites (Arief et al. 2008). Using higher adsorbent dose > 2 g/l caused deterioration of both parameters. This can be attributed either to repulsion between adsorbed oil particles on the surface of adsorbent and those still remaining in oil–water emulsion at equilibrium or to aggregation of adsorbent molecules causing, thereby reduction in the surface area (Sidik et al. 2012). The effect of adsorbent dose was studied at pH 8.0, polystyrene waste/surfactant modified bagasse weight ratio 1, initial emulsified oil concentration, 0.5 g/l for 30 min. The optimum adsorbent dose was found 2 g/l.
Freundlich (Freundlich, 1906) and Langmuir (Langmuir, 1916) isothermal models were applied to study the interaction between liquid and solid and also to depict the relationship between the amount of emulsified oil adsorbed and its equilibrium concentration.
The linear form of Freundlich isotherm is expressed by the following equation.
where qe is the amount of emulsified oil adsorbed at equilibrium (mg/g), Ce is the equilibrium concentration of the emulsified oil (mg/l), KF is the adsorption capacity of the adsorbate. The favorability of adsorption process is specified on the basis of n (adsorption intensity or surface heterogeneity). Both n and KF are constants. By plotting qe against Ce, 1/n and KF can be calculated from the slope and intercept of the straight line, respectively. These parameters are listed in Table 2. It was found that 1/n values between 0 and 1 means that oil adsorption process is heterogeneous and occurred through multilayer adsorption process.
Langmuir isotherm model (Eq. 7) suggests that the adsorption process occurs at homogeneous surface leading to monolayer coverage, basing on equal energy at all sites. b and qm are Langmuir constant and maximum adsorption capacity, respectively. By plotting Ce/qe versus Ce, qm and b can be determined from the intercept and slope of the straight line, respectively. RL is the essential factor and can be determined from Eq. 8. Higher value of RL (not lying between 0 and 1) indicates that adsorption process cannot fit Langmuir isotherm, besides, R2 and qm values confirmed this.
Different isothermal parameters including constants and correlation coefficient (R2) are listed in Table 3.
Thermodynamics studies
The changes in the standard Gibb’s free energy (ΔG°), enthalpy (ΔH°) and entropy (ΔS°) are identified as thermodynamics parameters, and used to describe the nature of oil adsorption process and their values can be detected from the following equations:
where Kc is the equilibrium constant of oil sorption and can be estimated from Eqs. 10 as follows:
where Csorbent and Cmixture are the concentrations of oil in the sorbent and the mixture at equilibrium, respectively.
where R is the ideal gas constant (8.314 J/mol K) and T is the absolute temperature (K).
The effect of temperature on oil adsorption capacity was studied under temperature range (298–333 K). As depicted in Table 4, increasing temperature from 298 to 313 K led to increase in oil adsorption capacity (qe) from 22.3 to 26.5 g/g but further increase to 333 K resulted in drastic decreasing in qe to 16.7 g/g. These results indicate endothermic behavior in temperature range (298–313 K) and exothermic behavior in the range (323–333 K).
The thermodynamics parameters ΔG°, ΔH° and ΔS° are calculated from Eqs. 9–11 at temperature range (298–333 K) and listed also in Table 4. It was noticed that ΔG° increased by increasing temperature from 298 to 313 K and decreased by increasing temperature to 333 K, the values of ΔG° are negatively assigned and this denotes that oil sorption process is spontaneous. The positive values of ΔH° and ΔS° confirmed endothermic and random oil sorption process.
Reusability studies
The efficiency of the reused blend to remove emulsified food oil several times from aqueous solutions was studied and carried out for 6 cycles. Figure 5b shows the effect of reusing the blend on oil removal efficiency. It was found that the oil removal efficiency (R %) of the reused blend remained constant for 4 cycles (98.7%), then decreased slightly in cycle 5, reached 97.6% and continued to decrease in cycle 6 to 90.3%.
Summary and conclusions
-
In the present work, polystyrene waste was blended with surfactant modified bagasse and the different samples were characterized by many techniques.
-
Compared with bagasse, FTIR spectra of surfactant modified bagasse and polystyrene waste/surfactant modified bagasse showed changes in the intensity and position of some peaks indicating the presence of the new moieties.
-
SEM images of the modified sample and blend exhibited different morphologies and XRD experiments confirmed the alteration of the highly crystalline bagasse to an amorphous structured blend.
-
Elemental analysis tests showed increasing C and H content upon modification, whereas N2 content increased in case of surfactant modified bagasse and kept constant after blending with polystyrene waste.
-
Modification of bagasse with CTAB led to reduced specific surface area due to the penetration of the surfactant into the pores of bagasse. On the other hand, specific surface area of the blend highly increased.
-
Also, modification of bagasse led to increasing hydrophobicity in the order: polystyrene waste/surfactant modified bagasse > surfactant modified bagasse > bagasse. All these measured properties give evidence of successful modification of bagasse.
-
To evaluate the modified samples as oleophilic adsorbents, the effect of varying experimental parameters on oil removal efficiency was investigated. Increasing polystyrene waste: surfactant modified bagasse weight ratio from 0.25 to 1 led to improvement in oil adsorption capacity and removal efficiency, whereas further increase to 2 was not necessary.
-
By using different initial oil concentrations, adsorption capacity increased until 2 g/l oil concentration. Higher concentrations, e.g., 3 g/l led to deteriorated adsorption capacity.
-
The effect of increasing contact time on oil removal efficiency was also studied. Maximum removal efficiency at equilibrium decreased by increasing oil concentration.
-
Initial pH of oily wastewater had also an important impact on oil adsorption capacity and the results showed that optimum oil adsorption capacity was reached at pH values 5.0 to 8.0. Also, the effect of adsorbent dose was investigated and found optimum at 2 g/l.
-
Reusability experiments till 5 cycles gave satisfactory results, isothermal models and thermodynamics studies were defined.
-
Generally, polystyrene waste/surfactant modified bagasse samples can be considered as a new valuable oil adsorbent.
References
Abdelwahab NA, Shukry N (2015) Synthesis, characterization and antimicrobial properties of grafted sugarcane bagasse/silver nanocomposites. Carbohydr Polym 115:276–284
Alayande SO, Dare EO, Msagati TAM, Akinlabi AK, Aiyedun PO (2016) Superhydrophobic and superoleophillic surface of porous beaded electrospun polystrene and polysytrene-zeolite fiber for crude oil-water separation. Phys Chem Earth 92:7–13
Ali N, El-harbawi M, Jabal AA, Yin C (2012) Characteristics and oil sorption effectiveness of kapok fibre, sugarcane bagasse and rice husks: oil removal suitability matrix. Environ Technol 33(4):481–486
Alther GR (2001) How to remove emulsified oil from wastewater with organoclays. Water Eng Manag HW Wilson AST 148:27–29
Aly AA, Mahmoud SA, El-Apasery MA (2018) Decolorization of reactive dyes, Part I: eco-friendly approach of reactive dye effluents decolorization using cationized sugarcane bagasse. Pigm Resin Technol 47(2):108–115
Arief VO, Trilestari K, Sunarso J, Indraswati N, Ismadji S (2008) Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: characterization, biosorption parameters and mechanism studies. CLEAN-Soil Air Water 36:937–962
Augusta P, Kalaichelvi P (2016) Investigation on microwave and ultrasound-assisted corn husk for the removal of emulsified engine oil from water. Desalin Water Treat 57:13120–13131
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
Bismarck A, Aranberri-Asargorta I, Springer J (2002) Surface characterization of flax, hemp and cellulose fibers; surface properties and the water uptake behavior. Polym Compos 23:872–894
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. Am Chem Soc 60:309–319
Cavdar AD, Mengeloglu F, Karakus K, Tomak ED (2014) Effect of chemical modification with maleic, propionic, and succinic anhydrides on some properties of wood flour filled HDPE composites. BioResources 9:6490–6503
Chai W, Liu X, Zou J, Zhang X, Li B, Yin T (2015) Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution. Carbohydr Polym 132:245–251
Chauhan RS, Gopinath S, Razdan P, Delattre C, Nirmala GS, Natarajan R (2008) Thermal decomposition of expanded polystyrene in a pebble bed reactor to get higher liquid fraction yield at low temperatures. Waste Manag Oxford 28:2140–2145
Chen M-J, Zhang X-Q, Liu C-F, Shi Q-S (2016) Homogeneous modification of sugarcane bagasse by graft copolymerization in ionic liquid for oil absorption application. Int J Polym Sci 2016:1–7
Chiparus OI (2004) Bagasse fiber for production of nonwoven materials. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, May
Chung S, Suidan MT, Venosa AD (2011) Partially acetylated sugarcane bagasse for wicking oil from contaminated wetlands. Chem Eng Technol 34:1989–1996
Cueva-Orjuelaa JC, Hormaza-Anaguanob A, Merino-Restrepo A (2017) Sugarcane bagasse and its potential use for the textile effluent treatment. DYNA 84(20):291–297
Demirbas E, Nas MZ (2009) Batch kinetic and equilibrium studies of adsorption of reactive blue 21 by fly ash and sepiolite. Desalination 243:8–21
Du C, Wang J, Chen Z, Chen D (2014) Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl Surf Sci 313:304–310
Dumore NS, Mukhopadhyay M (2012) Removal of oil and grease using immobilized triacylglycerin lipase. Int Biodeterior Biodegrad 68:65–70
Farah JY, El-Gendy NS, Farahat LA (2007) Biosorption of astrazone blue basic dye from an aqueous solution using dried biomass of Baker’s yeast. J Hazard Mater 148:402–408
Freundlich HMF (1906) Über dies adsorption in Lösungen. Zeitschrift für Physikalische Chemie. Int J Res Phys Chem Chem Phys 57:385–470
Fu YC, Li G, Yu HP, Liu YX (2012) Hydrophobic modification of wood via surface-initiated ARGET ATRP of MMA. Appl Surf Sci 258(7):2529–2533
Gupta R, Mauri R, Shinnar R (1999) Phase separation of liquid mixtures in the presence of surfactants. Ind Eng Chem Res 38:2418–2424
Hafshejani LD, Hooshmand A, Naseri AA, Mohammadi AS, Abbasi F, Bhatnagar A (2016) Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol Eng 95:101–111
Hallett JP, Welton T (2011) Room-temperature ionic liquids: solvents for synthesis and catalysis. 2. Chem Rev 111:3508–3576
Hearon K, Nash LD, Rodriguez JN, Lonnecker AT, Raymond JE, Wilson TS, Wooley KL, Maitland DJ (2014) A high-performance recycling solution for polystyrene achieved by the synthesis of renewable poly(thioether) networks derived from d-limonene. Adv Mater 26:1552–1558
Ibrahim S, Ang HM, Wang S (2009) Removal of emulsified food and mineral oils from wastewater using surfactant modified barley straw. Biores Technol 100:5744–5749
Ibrahim S, Wang S, Ang HM (2010) Removal of emulsified oil from oily wastewater using agricultural waste barley straw. Biochem Eng J 49(1):78–83
Kanwal S, Chaudhry N, Munir S, Sana H (2019) Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag 88:280–290
Kargarzadeh H, Ahmad I, Abdullah I, Dufresne A, Zainuddin SY, Sheltami RM (2012) Effects of hydrolysis conditions on the morphology, crystallinity and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 19(3):855–866
Kundu P, Mishra IM (2013) Removal of emulsified oil from oily wastewater (oil-in-water emulsion) using packed bed of polymeric resin beads. Sep Purif Technol 118:519–529
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Li J, Gu Y (2005) Coalescence of oil-in-water emulsions in fibrous and granular beds. Sep Purif Technol 42:1–13
Marković-Nikolić D, Bojić A, Petković G, Ristić N, Cakić M, Nikolić G (2017) The preparation and utilization of the cationic sorbent based on the surfactant modified bottle gourd shell. Adv Technol 6(2):38–50
Nazir MS, Bambang AW, Yussof AW, Abdullah A (2013) Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. BioResources 8(2):2161–2172
Pachathu A, Ponnusamy K, Srinivasan SKVR (2016) Packed bed column studies on the removal of emulsified oil from water using raw and modified bagasse and corn husk. J Mol Liq 223:1256–1263
Pan Y, Wang F, Wei T, Zhang C, Xiao H (2016) Hydrophobic modification of bagasse cellulose fibers with cationic latex: adsorption kinetics and mechanism. Chem Eng J 30:233–243
Said AEAA, Ludwick AG, Aglan HA (2009) Usefulness of raw bagasse for oil absorption: a comparison of raw and acylated bagasse and their components. Biores Technol 100(7):2219–2222
Sangal VK, Mishra IM, Kushwaha JP (2013) Electrocoagulation of soluble oil wastewater: parametric and kinetic study. Sep Sci Technol 48(7):1062–1072
Satirawaty A, Pauzan M, Ahad N (2018) Biomass modification using cationic surfactant cetyltrimethylammonium bromide (CTAB) to remove palm-based cooking oil. J Chem 2018:1–7
Shah J, Jan MR, Adnan (2014) Catalytic activity of metal impregnated catalysts for degradation of waste polystyrene. J Ind Eng Chem 20:3604–3611
Sidik SM, Jalil AA, Triwahyono S, Adam SH, Satar MAH, Hameed BH (2012) Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem Eng J 203:9–18
Song Y, Zhang L, Gan W, Zhou J, Zhang L (2011) Self-assembled micelles based on hydrophobically modified quarternized cellulose for drug delivery. Colloids Surf B Biointrefaces 83:313–320
Wahi R, Chuah LA, Ngaini Z, Nourouzi MM, Choong TSY (2014) Esterification of M. sagu bark as an adsorbent for removal of emulsified oil. J Environ Chem Eng 2:324–331
Wang J, Zheng Y, Wang A (2013) Investigation of acetylated kapok fibers on the sorption of oil in water. J Environ Sci 25(2):246–253
Wikipedia, “Bagasse,” 2015, https://en.wikipedia.org/wiki/Bagasse
Xie H, King A, Kilpelainen I, Granstrom M, Argyropoulos DS (2007) Thorough chemical modification of wood-based lignocellulosic materials in ionic liquids. Biomacromol 8(12):3740–3748
Yang H, Bian S, Hu J, Li F, Yao T (2016) Effect of water chemistry on the adsorption of lubricating oil on oxidized graphite. J Mol Liq 219:1157–1160
Zainuddina N, Ahmada I, Kargarzadeha H, Ramli S (2017) Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr Polym 163:261–269
Zhang N, Jiang W, Wang TH, Gu JJ, Zhong ST, Zhou S, Xie T, Fu JJ (2015) Facile preparation of magnetic poly(styrene-divinylbenzene) foam and its application as an oil absorbent. Ind Eng Chem Res 54:11033–11039
Zhang B, Dong Z, Sun D, Wu T, Li Y (2017a) Enhanced adsorption capacity of dyes by surfactant-modified layered double hydroxides from aqueous solution. J Ind Eng Chem 49:208–218
Zhang J, Xue Q, Pan X, Jin Y, Lu W, Ding D, Guo Q (2017b) Graphene oxide/polyacrylonitrile fiber hierarchical-structured membrane for ultra-fast microfiltration of oil-water emulsion. Chem Eng J 307:643–649
Zhou Y-B, Tang X-Y, Hu X-M, Fritschi S, Lu J (2008) Emulsified oily wastewater treatment using a hybrid-modified resin and activated carbon system. Sep Purif Technol 63:400–406
Zhou YB, Chen L, MengHu X, Lu J (2009) Modified resin coalescer for oil-in-water emulsion treatment: effect of operating conditions on oil removal performance. Ind Eng Chem Res 48:1660–1664
Zhou Y, Tang X, Xu Y, Lu J (2010) Effect of quaternary ammonium surfactant modification on oil removal capability of polystyrene resin. Sep Purif Technol 75:266–272
Acknowledgements
Financial support for this work was funded by the National Research Center through the Project No. 10130101. The authors express their deep appreciation to the National Research Center for this support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelwahab, N.A., Shukry, N. & El-kalyoubi, S.F. Separation of emulsified oil from wastewater using polystyrene and surfactant modified sugarcane bagasse wastes blend. Clean Techn Environ Policy 23, 235–249 (2021). https://doi.org/10.1007/s10098-020-01973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-020-01973-1