Abstract
Carbon dioxide emission, which acts as one of the major agents of greenhouse gases (GHG), has significant effects on global warming. Nowadays, there is a considerable global tendency toward decreasing the amount of GHG emissions to the atmosphere. In the present study, a simulated power plant flue gas (Be’sat, Power Plant, Tehran) with a constant injection rate of 21.41 cm3 s−1, including 10% CO2, 7% O2 and 83% N2 , was injected to the Synechococcus elongatus culture under two different light–dark (L/D) cycles: 24-0 and 16-8. Additionally, the biomass productivity and the CO2 biofixation rate by microorganisms were investigated. The highest biomass productivities were recorded as 0.68 and 0.52 g L−1 d−1 for 24-0 and 16-8 L/D cycles, respectively. Furthermore, the maximum rate of the CO2 biofixation was 1.26 g L−1 d−1 for the 24-0 L/D cycle and 0.98 g L−1 d−1 for the 16-8 L/D cycle during the cultivation.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are prokaryote (cyanobacteria) and eukaryote (common microalgae) photosynthetic microorganisms which can grow in harsh environmental conditions owing to their simple structures (Mata et al. 2010). The biomass produced from microalgae and cyanobacteria can be utilized as the feedstock for biofuels (de Morais and Costa 2007; Ho et al. 2011). Microalgae can grow up to 100 times faster than terrestrial plants. Their biomass can be duplicated in less than one day. Almost more than 50,000 microalgae species exist in aquatic and non-aquatic ecosystems, but approximately 30,000 species have been investigated (Mata et al. 2010; Tredici 2010). Phototrophic microalgae exploit CO2 as the inorganic carbon source and convert it to carbohydrate, which is used to build proteins, lipids, and nucleic acids. These high-value molecules can be converted to other valuable products such as biopolymer-based substances and biofuel. In addition, they can efficiently capture atmospheric CO2 and transform the solar energy into chemical energy up to 10–50 times more than other terrestrial plants do (Beer et al. 2009; Kassim et al. 2014; Khan et al. 2009; Li et al. 2008; Rosenberg et al. 2011).
Cyanobacteria are located in the bacterial domain in some classifications. They have a wide distribution around the world and have the photosynthetic ability and can fix a large amount of carbon dioxide in marine and freshwater environments (Steffen et al. 2012). Synechococcus elongatus is the rod shape freshwater oligotrophic photoautotroph unicellular cyanobacteria microorganism that can survive in low nutrients freshwater habitat. They can also live in freshwater hot springs with the medium temperature. Furthermore, cyanobacteria sometimes categorized as blue-green algae are the prokaryote cells with the photosynthetic ability to provide their required energy (Waterbury 1986). For biosynthesis and other energetic requirements, Synechococcus elongatus absorbs carbon dioxide and then transforms it into glucose through the Calvin cycle (Yang et al. 2003). Like most plants, chlorophyll a is a primary pigment in the Synechococcus elongatus photosynthesis process. Blue-green pigmentation in this species is related to phycobiliproteins as other cyanobacteria (Olson et al. 1990).
Microalgae need some nutrients for their growth, such as nitrogen and phosphorus, which also exist in common wastewater. Thus, microalgae can remove these nutrients from various effluents (Parmar et al. 2011). Some kinds of microalgae can accumulate considerable lipid globes within their cells that can be used for biofuel production (Milano et al. 2016). Additionally, microalgal biomass can be used to produce bio-fertilizers and biomethane (biogas) (Parmar et al. 2011; Saratale et al. 2018). Nowadays, accumulation and increase in the level of CO2 in the atmosphere causing global warming are one of the most significant challenges facing human beings. Some of the destructive impacts of global warming are: reducing and melting the Arctic ice, rising the water’s level of seas and oceans, huge perishing of sea coral, obstructing agricultural plans, and expanding the occurrence scale of desertification and droughts all over the world (Yang et al. 2012).
Hence, microalgae and photosynthetic cyanobacteria can be the promising choices to reduce the CO2 level in the atmosphere by the capturing process and even convert them to valuable compounds. Basically, microalgae can be classified as CO2-sensitive (2–5%) and CO2 tolerant (5–20%) regarding the tolerance of the CO2 concentration; therefore, selection of the proper microalgae strain for the CO2 biofixation process is an important issue that should be considered (Miyachi et al. 2003). Some evidence proves that some species of cyanobacteria and microalgae, compared to CO2 streams, may have better performance with flue gas (Douskova et al. 2009). Moreover, toleration of rational high temperatures (compared to typical photosynthetic organisms) is another fundamental characteristic that some species of cyanobacteria and microalgae have. The former specification to some extent can decrease the crucial need for declining the flue gas temperature before contact (Umar et al. 2019).
Two conventional methods exist for the massive cultivation of microalgae, namely open systems and closed cultivation systems. These systems have their advantages and disadvantages. Open systems are relatively cheaper, but they are highly susceptible to environmental contaminations and can deeply be affected by climate changes and weather conditions. Although closed systems can overcome these drawbacks, they are expensive concerning their construction, maintenance, and operation (Cheah et al. 2015; Hashemi et al. 2020). Generally, approximately 70% of CO2 biofixation projects employing microalgae and cyanobacteria as photosynthetic microorganisms utilize open pond systems for their objectives (Umar et al. 2019). These systems that utilize natural sunlight can be more efficient in term of electricity consumption and lower CO2 emission, but it should be considered that the total productivity of these systems is not as high as that of closed systems. Moreover, closed systems require more energy to provide desirable mixing, illumination, and CO2 mass transfer through cultivation (Slade and Bauen. 2013).
A number of researches use cyanobacteria and microalgae in the biological CO2 removal process. Sadeghizadeh et al. (2017) investigated the effect of superficial gas velocity on the growth pattern and CO2 removal of Chlorella vulgaris species in an airlift photobioreactor. The results revealed 80% CO2 biofixation achieved in the superficial gas velocity of 7.458 × 10−3 ms−1. Arroyo et al. (2019) employed three airlift bioreactors (in series) to cultivate Scenedesmus dimorphus under 24-0, 16-8, and 14-10 L/D cycles. They obtained the highest CO2 removal of 64.3% under 60.75 µmol photons m−2 s−1 and 16-8 L/D cycles. Mortezaeikia et al. (2016) investigated the effects of membrane contactor, and membrane-sparger photobioreactors on the CO2 biofixation with the Synechococcus elongatus species in various carbon dioxide concentrations (0.04%, 5%, and 10%). The results demonstrated that the highest carbon dioxide removal of 2.43 and 2.08 g L−1 d−1 was recorded within membrane-sparger and membrane contactor in the carbon dioxide concentrations of 5% and 10%, respectively. Barahoei et al. (2020) cultured the Chlorella vulgaris species within a bubble column photobioreactor and assessed the effect of air bubbles on the CO2 biofixation rate. The maximum carbon dioxide biofixation rate and biomass productivity were obtained for smaller gas feeding bubble diameters enriched by 7% CO2 concentration as approximately 633.73 mg L−1 d−1, 337 mg L−1 d−1, respectively.
In the present paper, a simulated power plant (Be’sat, Tehran) flue gas, including 10% CO2, was performed. The impact of this simulated flue gas on Synechococcus elongatus species cultivation under 24-0 and 16-8 L/D cycles and the required time for the total CO2 removal were investigated, and some key parameters in CO2 biofixation and microorganisms growth were calculated and discussed.
Materials and methods
Organisms and medium
Synechococcus elongatus species that was purified from the hot spring of Ramsar (a northern city of Iran) was used in this study. The BG-11 standard culture medium was prepared for the proper growth of this species (Chinnasamy et al. 2010).
Culture conditions
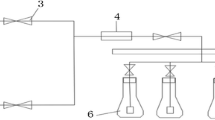
Synechococcus elongatus samples in an appropriate culture medium at an average temperature of 25 ± 2 °C and 54 µmol photons m−2 s−1 white light intensity of LEDs were cultivated. A gas stream with a constant injection rate (21.41 cm3 s−1) simulated to the flue gas of power plant of Be’sat (in Tehran), containing 10% CO2, 7% O2, and 83% N2 was continuously injected into each sample during the cultivation period. Continuous aeration with the rate of 73.08 cm3 s−1 was performed for each sample. Two L/D cycles, including 24-0 (24 h of white light radiation without any darkness period) and 16-8 (16 h white light radiation versus 8 h darkness), were presented, and their effects on different concerned parameters evaluated in this study were investigated. The aeration was stopped precisely 2 h after the beginning of the light radiation period, and one hour later, the trapped air was gathered by the gas basket for each sample. The effect of these L/D cycles on CO2 removal from the simulated power plant flue gas by the photosynthetic activity of this species and various parameters such as growth pattern, pH fluctuations (were recorded with 827 pH Lab, Switzerland) chlorophyll a and b production, biomass productivity, and CO2 fixation rate were investigated. All the experiments were conducted in duplicate. Figure 1 presents the schematic of the system.
Analytical methods
To measure chlorophyll a and b, the extraction by the solvent (methanol) method was used (Delavari Amrei et al. 2014):
All absorbance values (Optical density) were detected at the 645 nm and 662 nm wavelength via a spectrophotometer device (T80 + UV–Vis, United Kingdom). The percentage of the CO2 content within the gas was determined by the GC–MS (Agilent 6890 N, USA) method. The dry weight values were obtained with the method employed by Delavari Amrei et al. (2014).
Growth kinetics and CO2 biofixation
Biomass productivity was calculated by the following equation:
P (g L−1 d−1) is the biomass productivity, X0 (g L−1) is the initial cell concentration, and Xf (g L−1) is the final cell concentration at the time of tf,
The CO2 consumption (biofixation) rate PCO2 (g L−1 d−1) was calculated with the following equation (Kassim and Meng 2017):
MCO2 and MC are the molecular weight of carbon dioxide and carbon, respectively. Cc is the average carbon content in dry biomass. The typical molecular chemical formula of microalgae biomass is CO0.48H1.83N0.11P0.01. Based on this formula, the carbon content (Cc) is approximately 50%, and the CO2 fixation rate correlation is summarized as follows (Chisti 2007):
According to Eq. 5 with 1 kg of biomass production during the carbon biofixation process, approximately 1.8 kg CO2 can be captured.
Energy balance
In this survey, the equivalent acceptable energy consumption (kWh) for one liter of Synechococcus elongatus culture during one day is calculated. The preset setup or every other system with this carbon biofixation rate has to work below this range to be acceptable in positive energy balance. These statistics were obtained from the United States Environmental Protection Agency website. Furthermore, some other factors (biofuel production, potential of wastewater treatment, and produced biomass) that can affect the energy balance of the process are discussed. These factors can play a compensative role in the energy consumption issue related to the CO2 removal process.
Results and discussion
Photosynthetic microorganisms such as microalgae and cyanobacteria have the considerable ability to capture CO2 molecules and convert it to the high-value organic substances with the help of light energy radiation and production of molecular oxygen as a by-product using the following reaction (Zhang 2015):
Microorganisms can capture CO2 from various sources, including CO2 biofixation from the atmosphere, industrial discharge flue gas, and dissolved carbonates within the culture (Lam et al. 2012). Typically, carbon dioxide concentration within industrial flue gases is between 10 and 20% (Ho et al. 2011). According to the literature, an excessive amount of CO2 in the culture aeration flow can actuate the microalgae cells to grow faster (Chiu et al. 2008; Jiang et al. 2011; Tang et al. 2011). As Fig. 2 shows, the 24-0 L/D cycle had better growth performance than that of the 16-8 L/D cycle. Desired cell growth in the 24-0 cycle could be due to a longer light period. Originally, photosynthesis is categorized as a physicochemical process and comprises light-dependent and light-independent reactions. Light-dependent reactions occur only in the presence of light, and light-independent reactions are accomplished in light or darkness (Zhang 2015). The light-dependent reactions occur within the chloroplast of cells (thylakoid membranes), and NADPH2 (nicotinamide adenine dinucleotide hydrogen phosphate: acts as an electron carrier), ATP (Adenosine Triphosphate: the primary energy carrier in all living organisms on the earth), and O2 are produced in this stage. In addition, light-independent interactions occur within the stroma of the chloroplast. The decrease in the CO2 concentration due to biofixation is the result of these interactions in this step (Martín-Girela et al. 2017). The dissolved NO of the flue gas can be consumed by microalgae as an alternative nitrogen source, which can be oxidized to nitrate or nitric within the culture (Nagase et al. 2001). Three kinds of inorganic carbon can be formed when CO2 molecules enter into and dissolve in water, depending on the pH of the solution: free CO2 molecules and H2CO3 are the dominant in the pH less than 4.5, HCO3− is dominant species in the pH range of 4.5 to 8.5, and in the pH range of more than 8.5, and the CO3 2− is the dominant species in the solution (Devgoswami et al. 2011).
According to Fig. 3, the highest pH value for 16-8 and 24-0 L/D cycles was on the 5th and 4th day of cultivation, respectively. Basically, the pH descending procedure between the initial time (time: 0) and the first days of cultivation could be attributed to the CO2 accumulation within the culture due to low cell density and low cell photosynthesis and subsequently low CO2 consumption. Microalgal species have their own pH range for optimum growth conditions. An ascending procedure in pH values during the cultivation period (alkalinization of the medium) is related to the photosynthetic activities of cells. This common phenomenon occurs due to CO2 consumption by cells, leading to decrease in CO2 concentrations and accumulation of hydroxide ions (Shiraiwa et al. 1993). Furthermore, the decrease in the pH value is contributed to the formation of dissolved inorganic carbon, presence of hydrogen cations within the culture, and constitution of H2CO3, HCO3−, and CO32− ions (as a result of interaction between CO2 and H2O molecules) (de Morais and Costa 2007). A reduction in photosynthetic enzyme (such as carbonic extracellular anhydrase) activities occurs as a result of the decrease in the pH value of the culture, and this can cause the inhabitation in the microalgal growth (Tang et al. 2011).
As Fig. 4 illustrates the minimum required time for the CO2 removal (100%) from the simulated power plant flue gas (10% CO2 content) was recorded for the culture with the 24-0 L/D cycle 7 days after cultivation, and this value was 8 days for the 16-8 L/D cycle. This could be related to longer light exposure for the 24-0 L/D cycle, which could cause more photosynthetic activity by the cyanobacteria species. Obviously, the more photosynthetic activity is, the more CO2 consumption for the production of organic compounds within microorganism cells is. From another point of view, the rate of the CO2 consumption in the 24-0 L/D cycle was more than that in the 16-8 L/D cycle. This could be the other corroborative deduction for the higher photosynthetic rate in the longer irradiation cycle. The CO2 fixation rate and light irradiation intensity are the main factors for the growth of photosynthetic microorganisms (Stewart and Hessami 2005). Yun et al. (1997) studied the impact of different CO2 concentrations on Chlorella vulgaris and reported that a 15% CO2 enriched aeration had an inhibitive effect on the growth of this microalgae species, while the best growth pattern was in 5% CO2. Adamczyk et al. (2016) investigated the kinetic growth of Chlorella sp. under 4 and 8% CO2 enriched aeration and found that the 8% CO2 had a better impact on the growth pattern of Chlorella sp. Yoo et al. (2010) tested different species under direct flue gas. According to their results, Botryococcus braunii and Scenedesmus sp. demonstrated the highest growth rate in flue gas, including 5.5% CO2. Li et al. (2011) stated that Scenedesmus obliquus could tolerate up to 12% CO2 concentration in industrial flue gas by the 67% CO2 removal during the process. Maeda et al. (1995) conducted some experiments on CO2 removal from the coal-fired power plant with the Chlorella sp. Their results revealed that the highest growth rate was achieved by 10% CO2.
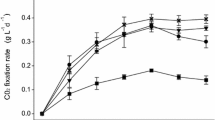
As the previous section showed, the biomass productivity was calculated by Eq. 1. Based on Fig. 5, the highest biomass productivity was recorded as 0.68 (g L−1 d−1) on the 6th day of cultivation for 24-0 and 0.52 (g L−1 d−1) on the 5th for the 16-8 L/D cycle. The CO2 fixation rate was estimated by Eqs. 2 and 3. As Fig. 6 illustrates the highest CO2 consumption rate was 1.26 g L−1 d−1 on the 6th day of the cultivation process for the 24-0 L/D cycle and 0.98 g L−1 d−1 for the 16-8 L/D cycle on day 5. Based on the results, the day with the highest biomass productivity and the highest CO2 biofixation were exactly the same for each related L/D cycle. These results obviously revealed that the more photosynthetic activity was, the more biomass productivity, and the more CO2 fixation rate were. De Morais and Costa (2007) examined the growth characteristics of Scenedesmus obliquus and Chlorella kessleri with the exposure of 6% and 12% CO2. The maximum biomass productivities were 0.087 g L−1 d−1 for 6% CO2 concentration and 1.14 g L−1 d−1 for Scenedesmus obliquus in 12% CO2 concentration. Some Chlorella and cyanobacteria can depict a favorable growth pattern under high CO2 concentration with high CO2 biofixation capability (500-1800 mg L−1 d−1) (Maeda et al. 1995; Ono and Cuello 2007; Yue and Chen 2005). Zhao and Su (2014) conducted one study on Chlorella sp., which had promising performance in CO2 fixation by 1.060 g L−1 d−1 biomass productivity and 1.992 g L−1 d−1 carbon fixation rate. Among the microalgae species, Chlorella sp., Scenedesmus sp., and Botryococcus braunii have exhibited high performance in CO2 mitigation with the CO2 fixation rate of 0.2–1.3 g L−1 d−1 (Lam and Lee 2012).
Based on Fig. 7, the highest chlorophyll a to cell dry weight ratio (Chl a/DW) for 24-0 and 16-8 L/D cycles was reported on the fifth day of cultivation as 1.42 and 1.34 mg chlorophyll per gram of cell dry weight, respectively. Furthermore, their basic fluctuations were in accordance with each other. On day five (the maximum value of Chl a/DW), the cells at 24-0 and 16-8 L/D cycles were in the middle of the logarithmic phase of growth. Additionally, Fig. 8 illustrates the highest ratios of chlorophyll b to cell dry weight (Chl b/DW) were 2.37 and 3.34 (mg g−1) on the third day of cultivation for 24-0 and 16-8 L/D cycle, respectively. These fluctuations began descending mode after the third day of cultivation, and declined to their minimum level on day 8.
Carbonic anhydrase enzyme has the essential function on the surface of the cell by converting HCO3− to CO2 and simplifying the transportation of CO2 into the cell. Generally, passing HCO3− through the chloroplast membrane is impossible (Tsuzuki and Miyachi 1989). When the CO2 supply of the cultivation system has the limitation of providing required CO2 for the photosynthesis of microorganisms, the role of carbonic anhydrase, which can aid the cells to synergize the carbon concentrating mechanism, becomes more crucial (Cannon et al. 2010). From another point of view, microalgal growth and their CO2 biofixation capability are closely associated with the amount of CO2 within the culture medium. An inhibitory state occurs in carbonic anhydrase activities in high CO2 concentration, and a significant increase in HCO3− and H+ concentrations and a decrease in the pH value of the medium occur. These interactions can weaken the carbon biofixation capability of microalgae cells. In this chronic situation, some microalgae species can adapt to these environmental changes by regulating genes, increasing adenosine triphosphate (ATP) production, and decreasing the synthesis of organic carbon to preserve the cells’ internal condition against pH reduction (Zhao and Su 2014).
Energy balance in CO2 emission and CO2 biofixation
According to the previous parts, the highest CO2 biofixation rate was 1.26 g L−1 d−1 for the 24-0 L/D cycle. Based on the United States Environmental Protection Agency, for 1 kWh electricity production, almost 1.559 lbs (707.149 g) CO2 was emitted to the atmosphere (https://www.epa.gov/, accessed on 20 January 2019). Therefore, simple calculations indicate that the present was simulated for Be’sat Power Plant flue gas or any other systems with this biofixation rate should consume less than 0.001788 kWh (1.788 Wh) per one liter of Synechococcus elongatus culture during one day to maintain its positive efficiency through the CO2 biofixation process.
As mentioned above, cyanobacteria and microalgae need light irradiation for their growth in both closed and open pond cultivation systems (Chisti 2007). Generally speaking, if every cultivation system can replace the artificial light with the outdoor natural light, or can reduce the energy demands for the mixing or aeration process, the total electricity consumption and the corresponding CO2 emission can be decreased significantly (Singh and Dhar. 2019; Slade and Bauen. 2013; Umar et al. 2019). Additionally, there are several species of microalgae that can accumulate a large amount of lipid in their cells under specific culture conditions. This produced biomass can be used for biofuel production that can be an effective factor in the regular energy balance in the biological CO2 capture process. Meanwhile, these microorganisms can assimilate nutrients such as nitrogen and phosphorus from different wastewater resources (secondary treatment). These nutrients uptake is the initial requirement for the growth of microalgae and cyanobacteria. If the procedures of CO2 biofixation and biological wastewater treatment can be accomplished simultaneously, it can lead to a decline in energy consumption. These factors should also be considered for every precise energy balance calculation. However, in this paper, the only concentration was on the net carbon dioxide that can be emitted in electricity production (for the energy demand of the present system), and the amount that can be directly captured by Synechococcus elongatus to form an initial balance in the calculations.
Conclusion
Cyanobacteria and microalgae have considerable potential for CO2 mitigation from atmospheric air and flue gas to reduce the impact of the CO2 (an important greenhouse gas) emission through the environment. A simulated flue gas of the power plant with 10% CO2 content was injected into the culture of Synechococcus elongatus. The best growth performance was for the 24-0 L/D cycle, and the microalgae could completely remove the CO2 content (100%) during seven days of cultivation at this L/D cycle, and the total CO2 removal lasted eight days at 16-8 L/D cycle. The ability of faster CO2 removal of the 24-0 cycle could be due to the longer duration of light irradiation resulting in more photosynthetic activity compared to the 16-8 cycle. The maximum Chl a/DW ratio for both 24-0 and 16-8 L/D cycles occurred on the 5th day of cultivation; however, the ratio of the 24-0 cycle was higher than that of the other cycle. Additionally, the maximum values of Chl b/DW obtained on the 3rd day of cultivation. However, this ratio in the 16-8 cycle was higher than the 24-0 cycle. The maximum CO2 consumption rate was for the 24-0 L/D cycle, indicating a better growth pattern and higher photosynthetic efficiency in the 24-0 L/D cycle. During this study, it was evidently demonstrated that the more photosynthetic activity was raised, the biomass productivity and the CO2 mitigation rate were increased.
References
Adamczyk M, Lasek J, Skawińska A (2016) CO2 biofixation and growth Kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl Biochem Biotech 179:1248–1261
Arroyo CA, Contreras JL, Zeifert B, Ramírez C (2019) CO2 capture of the gas emission, using a catalytic converter and Airlift Bioreactors with the Microalga Scenedesmus dimorphus. Appl. Sci. 9:3212
Barahoei M, Hatamipour MS, Afsharzadeh S (2020) CO2 capturing by chlorella vulgaris in a bubble column photo-bioreactor; Effect of bubble size on CO2 removal and growth rate. J. Co2 Util 37:9–19
Beer LL, Boyd ES, Peters JW, Posewitz MC (2009) Engineering algae for biohydrogen and biofuel production. Curr Opin Biotech 20:264–271
Cannon GC, Heinhorst S, Kerfeld CA (2010) Carboxysomal carbonic anhydrases: structure and role in microbial CO2 fixation. Biochim Biophys Acta Proteins Proteom 1804:382–392
Cheah WY, Show PL, Chang J-S, Ling TC, Juan JC (2015) Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour Technol 184:190–201
Chinnasamy S, Bhatnagar A, Hunt RW, Das K (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101:3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chiu S-Y, Kao C-Y, Chen C-H, Kuan T-C, Ong S-C, Lin C-S (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396
De Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445
Delavari Amrei H, Nasernejad B, Ranjbar R, Rastegar S (2014) An integrated wavelength-shifting strategy for enhancement of microalgal growth rate in PMMA-and polycarbonate-based photobioreactors. Eur J Phycol 49:324–331
Devgoswami CR, Kalita M, Talukdar J, Bora R, Sharma P (2011) Studies on the growth behavior of Chlorella, Haematococcus and Scenedesmus sp. in culture media with different concentrations of sodium bicarbonate and carbon dioxide gas. Afr J Biotechnol 10:13128–13138
Douskova I, Doucha J, Livansky K, Machat J, Novak P, Umysova D, Zachleder V, Vitova M (2009) Simultaneous flue gas bioremediation and reduction of microalgal biomass production costs. Appl Microbiol Biotechnol 82:179–185
Hashemi A, Moslemi M, Pajoum Shariati F, Delavari Amrei H (2020) Beta-carotene production within Dunaliella salina cells under salt stress condition in an indoor hybrid helical-tubular photobioreactor. Can J Chem Eng 98:69–74
Ho S-H, Chen C-Y, Lee D-J, Chang J-S (2011) Perspectives on microalgal CO2-emission mitigation systems—a review. Biotechnol Adv 29:189–198. https://www.epa.gov/. Accessed on 20 January 2019
Jiang L, Luo S, Fan X, Yang Z, Guo R (2011) Biomass and lipid production of marine microalgae using municipal wastewater and high concentration of CO2. Appl Energ 88:3336–3341
Kassim MA, Meng TK (2017) Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production. Sci Total Environ 584:1121–1129
Kassim M, Potumarthi R, Tanksale A, Srivatsa S, Bhattacharya S (2014) Enzymatic saccharification of dilute alkaline pre-treated microalgal (Tetraselmis suecica) biomass for biobutanol production. Int J Biol Vet Agric Food Eng 6:967–972
Khan SA, Hussain MZ, Prasad S, Banerjee U (2009) Prospects of biodiesel production from microalgae in India. Renew Sust Energ Rev 13:2361–2372
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690
Lam MK, Lee KT, Mohamed AR (2012) Current status and challenges on microalgae-based carbon capture. Int J GreenH Gas Con 10:456–469
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biofuels from microalgae. Biotechnol Prog 24:815–820
Li F-F, Yang Z-H, Zeng R, Yang G, Chang X, Yan J-B, Hou Y-L (2011) Microalgae capture of CO2 from actual flue gas discharged from a combustion chamber. Ind Eng Chem Res 50:6496–6502
Maeda K, Owada M, Kimura N, Omata K, Karube I (1995) CO2 fixation from the flue gas on coal-fired thermal power plant by microalgae. Energ Convers Manag 6:717–720
Martín-Girela I, Curt MD, Fernández J (2017) Flashing light effects on CO2 absorption by microalgae grown on a biofilm photobioreactor. Algal Res 25:421–430
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14:217–232
Milano J, Ong HC, Masjuki H, Chong W, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sustain Energy Rev 58:180–197
Miyachi S, Iwasaki I, Shiraiwa Y (2003) Historical perspective on microalgal and cyanobacterial acclimation to low-and extremely high-CO2 conditions. Photosynth Res 77:139–153
Mortezaeikia V, Yegani R, Tavakoli O (2016) Membrane-sparger vs. membrane contactor as a photobioreactors for carbon dioxide biofixation of Synechococcus elongatus in batch and semi-continuous mode. J cO2 Util 16:23–31
Nagase H et al (2001) Uptake pathway and continuous removal of nitric oxide from flue gas using microalgae. Biochem Eng J 7:241–246
Olson RJ, Chisholm SW, Zettler ER, Armbrust EV (1990) Pigments, size, and distributions of Synechococcus in the North Atlantic and Pacific Oceans. Limnol Oceanogr 35:45–58
Ono E, Cuello J (2007) Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst Eng 96:129–134
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–10172
Rosenberg JN, Mathias A, Korth K, Betenbaugh MJ, Oyler GA (2011) Microalgal biomass production and carbon dioxide sequestration from an integrated ethanol biorefinery in Iowa: a technical appraisal and economic feasibility evaluation. Biomass Bioenerg 35:3865–3876
Sadeghizadeh A, Moghaddasi L, Rahimi R (2017) CO2 capture from air by Chlorella vulgaris microalgae in an airlift photobioreactor. Biores Technol 243:441–447
Saratale RG, Kumar G, Banu R, Xia A, Periyasamy S, Saratale GD (2018) A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour Technol 262:319–332
Shiraiwa Y, Goyal A, Tolbert N (1993) Alkalization of the medium by unicellular green algae during uptake dissolved inorganic carbon. Plant Cell Physiol 34:649–657
Singh J, Dhar DW (2019) Overview of carbon capture technology: microalgal biorefinery concept and state-of-the-art. Front Mar Sci 6:29
Slade R, Bauen A (2013) Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 53:29–38
Steffen MM, Li Z, Effler TC, Hauser LJ, Boyer GL, Wilhelm SW (2012) Comparative metagenomics of toxic freshwater cyanobacteria bloom communities on two continents. PLoS ONE 7:e44002
Stewart C, Hessami M-A (2005) A study of methods of carbon dioxide capture and sequestration—the sustainability of a photosynthetic bioreactor approach. Energ Convers Manag 46:403–420
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Tsuzuki M, Miyachi S (1989) The function of carbonic anhydrase in aquatic photosynthesis. Aquat Bot 34:85–104
Umar A, In-na P, Wallace AD, Flickinger M, Caldwell GS, Lee J (2019) Loofah-based microalgae and cyanobacteria biocomposites for intensifying carbon dioxide capture. Available at SSRN 3489079
Waterbury JB (1986) Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Photosynthetic picoplankton:71-120
Yang M, Damjanović A, Vaswani HM, Fleming GR (2003) Energy transfer in photosystem I of cyanobacteria Synechococcus elongatus: model study with structure-based semi-empirical Hamiltonian and experimental spectral density. Biophys J 85:140–158
Yang C-Y, Fang Z, Li B, Long Y-f (2012) Review and prospects of Jatropha biodiesel industry in China. Renew Sust Energ Rev 16:2178–2190
Yoo C, Jun S-Y, Lee J-Y, Ahn C-Y, Oh H-M (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:S71–S74
Yue L, Chen W (2005) Isolation and determination of cultural characteristics of a new highly CO2 tolerant fresh water microalgae. Energ Convers Manage 46:1868–1876
Yun YS, Lee SB, Park JM, Lee CI, Yang JW (1997) Carbon dioxide fixation by algal cultivation using wastewater nutrients. J Chem Technol Biotechnology: International Research in Process, Environ Clean Tech 69:451–455
Zhang X (2015) Microalgae removal of CO2 from flue gas clean coal technology research reports, April Retrieved from http://bookshop.iea-coalorg.uk/reports/ccc-250/83697.244
Zhao B, Su Y (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sust Energ Rev 31:121–132
Acknowledgements
The authors would like to sincerely thank Parisa and Mahsa Keyvan Hoseini for editing the manuscript through the native English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashemi, A., Pajoum Shariati, F., Sohani, E. et al. CO2 biofixation by Synechococcus elongatus from the power plant flue gas under various light–dark cycles. Clean Techn Environ Policy 22, 1735–1743 (2020). https://doi.org/10.1007/s10098-020-01912-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-020-01912-0