Abstract
Organic pollutants cause serious impacts on environment. A novel method to synthesize ZnO/Ag–Ag2O microstructures was developed for organic pollutant photocatalytic degradation. The 3D pompon-like Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were firstly synthesized in a creative way by combining reaction in ethanol/water mixing layer and room-temperature self-assembly together. The length of nanosheets on Zn(OH)1.68(SO4)0.16·0.58H2O microspheres was controlled from ~ 0.8 to ~ 4.4 μm successfully. The method reduced both the processing temperature and reaction time compared with conventional ripening, which shortened catalysts’ production cycle, reduced production cost and simplified production condition. It holds great potential for industrial application. As traditional nanocatalysts are not easy to recycle and the residual nanocatalysts are also potential danger to the environment, our microsized ZnO/Ag–Ag2O photocatalysts inherit advantages of both nanoparticles and microspheres with enhanced removal efficiency and excellent recycle ability. Ag–Ag2O nanoparticles in ZnO/Ag–Ag2O microstructures were used to enhance the photocatalytic performance. 83.2% and 96.3% degradation efficiencies of Congo red were achieved by pure ZnO and ZnO/Ag–Ag2O microstructures after 80 min irradiation by UV light. 78.2% and 95.6% degradation efficiencies of methylene blue were achieved by pure ZnO and ZnO/Ag–Ag2O after 40 min under UV radiation. The degradation rate constants by ZnO/Ag–Ag2O microstructures are 0.04056 min−1 for Congo red and 0.07629 min−1 for methylene blue respectively. The ZnO/Ag–Ag2O photocatalyst showed excellent stability and reusability, since it was reused at least 5 times without any extra treatment. The ZnO/Ag–Ag2O microstructures may hold potential for organic pollutant treatments.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the rapid growth of economy and population, water contaminations, especially organic pollutants, have become a serious issue all over the world. Congo red is a popular benzidine-based anionic bisazo dye used in many industries. It is regarded as a typical representative of high-toxicity and hard-degradation organic pollutant in water treatment. So far, many strategies were proposed for organic pollutant treatment. Among them, the photocatalysis method has been regarded as an environmental-friendly, low-cost and sustainable process (Lee et al. 2013). During the photocatalytic process, the photocatalysts can capture photons with energy equal to or larger than their band gap energy to form electrons and holes on the surface of catalysts. It is considered that the superoxide and hydroxyl radicals are generated through the reaction between holes or electrons and water molecules to oxidize organic pollutants (Fu et al. 2015). The photocatalysts can be classified into two generic groups according to their different photocatalytic property in spectral region: ultraviolet (UV)- and visible-light-responsive photocatalysts. Many photocatalysts were reported for Congo red removal under visible-light irradiation. The chitosan/CdS (1.5 g L−1) removed 85.9% of Congo red (20 mg L−1) under a 300 W xenon lamp for 180 min (Zhu et al. 2009a). And 0.5 g L−1 of SrFe12O19 was used to remove 90% of Congo red (13.9 mg L−1) under a 125 W high-pressure Hg lamp for 180 min (Mohanta et al. 2013). For Ag2O/Ag2CO3, only 0.8 g L−1 of catalyst was mixed with higher concentration of Congo red (80 mg L−1) under a 500 W metal halide lamp (with an ultraviolet cutoff filter) for 20 min to reach 87.5% degradation (Zhao et al. 2017). Compared with visible-light irradiation, UV irradiation showed higher Congo red degradation effect. The dye degradation of 94% (Congo red, 10 mg L−1) was obtained by ZnS–Co2+ catalysts (0.8 g L−1) under UV irradiation from a 100 W low-pressure mercury-vapor lamp (Pouretedal et al. 2010). The higher degradation efficiency of 95.2% was achieved by the WO3–TiO2/AC catalysts (10 g L−1) for 40 mg L−1 of Congo red with a 500 W high-pressure mercury-vapor lamp illuminating for 2 h (Sun et al. 2009).
Recently, ZnO-based semiconductor photocatalysts with wide band gap, excellent chemical and physical stability and optoelectronic property have been regarded as promising materials for degrading organic dyes (Wang et al. 2018). Different approaches have been used for the preparation of ZnO, including solvothermal method (Guruvammal et al. 2016), solgel method (Ciciliati et al. 2015) and self-assembly method (Guo et al. 2015). Among them, self-assembly approach at room temperature attracted great attention due to the low-cost and mild reaction characteristics (Sau and Murphy 2004). Various methods were employed to improve the catalytic activity of ZnO via controlling its morphology (Chen et al. 2017), doping other chemical elements (Jesudoss et al. 2016), loading noble metal particles (Zhang et al. 2010), etc. Ag (Sampaio et al. 2017) and Ag2O (Yan et al. 2016) were reported as popular items in composite materials for ZnO photocatalysis to increase the UV–visible light adsorption intensity as well as the light adsorption range. The Ag–Ag2O composites can be a good choice for ZnO to enhance the photocatalytic ability.
The previous photocatalysts still hold some drawbacks, such as complex synthesis process, hard recycle and low degradation rate. Most photocatalysts with strict operation condition and high manufacturing cost would block their further industrial application. A simple and green method is necessary for preparing high-efficient photocatalysts. As the normal nanoparticles are not effective for recovery from water treatment process, microsized ZnO spheres can be a better choice, and with the help of nanosized Ag–Ag2O composites, the photocatalysts also possess special properties of nanomaterials. Most importantly, the improvement in photocatalysis performance is always the challenge for organic wastewater treatment. ZnO/Ag–Ag2O microspheres with high-efficient photocatalytic capability can be a better choice for future industrial applications.
Herein, we successfully fabricated a novel ZnO/Ag–Ag2O microstructure by a simple and green method as photocatalysts for Congo red degradation. The reaction at ethanol/water mixing layer was used to form Zn(OH)1.68(SO4)0.16·0.58H2O microspheres coated with nanosheets. The length of Zn(OH)1.68(SO4)0.16·0.58H2O nanosheets can be controlled by the reaction time. The combination of reaction at ethanol/water mixing layer and room-temperature Ostwald ripening was firstly developed to prepare 3D pompon-like Zn(OH)1.68(SO4)0.16·0.58H2O microspheres. After thermal treatment, the 3D pompon-like ZnO spheres were prepared. 3D pompon-like ZnO microspheres were decorated with Ag–Ag2O nanoparticles through reduction in silver nitrate. Coupling with Ag–Ag2O nanoparticles, the photocatalytic activity of 3D pompon-like ZnO microspheres for Congo red was enhanced by 13.1%. The ZnO/Ag–Ag2O microstructures may find great application in organic pollutant treatments.
Materials and methods
Materials
Zinc sulfate monohydrate, ammonium hydroxide solution, ethanol, silver nitrate, sodium borohydride, barium sulfate and Congo red were purchased from Sigma-Aldrich (China). All chemicals were used without any further purification. Ultrapure water (18.2 MΩ cm) was used for solution preparation in all experiments.
Preparation of 3D pompon-like ZnO spheres
The zinc sulfate monohydrate powders (0.1414 g) were mixed with 100 mL ethanol under ultrasonication for 2 h to form a crystal suspension. The diluted ammonium hydroxide solution (100 mL, 0.16 M) was dropped into the crystal suspension by violent shaking for 1 min. The white precipitations were formed immediately. The 3D pompon-like Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were formed after 75 min. To make sure the reaction is complete, the reaction time was increased to 6 h. The Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were washed by water and ethanol using low-speed centrifugation for three times, followed by drying in a vacuum oven at 60 °C. The 3D pompon-like Zn(OH)1.68(SO4)0.16·0.58H2O microspheres became ZnO products after heating them at 800 °C for 8 h.
Preparation of ZnO/Ag–Ag2O nanocomposites
As-synthesized ZnO products (0.5 g) were added into 50 mL AgNO3 solution (2.3 mM) under gentle stirring for 30 min. Then the freshly prepared NaBH4 (0.5 mL, 0.46 M) was added into the mixture for further 6 h under stirring. The productions were washed by low-speed centrifugation for three times and dried in a vacuum oven. The ZnO/Ag–Ag2O microstructures were formed successfully.
Adsorption ability and photocatalysis activity measurements
In adsorption experiments, 40 mg of catalysts was dispersed in 50 mL Congo red (40 mg L−1) aqueous solution in a quartz tube. At certain time intervals (0, 5, 10, 20, 30 and 40 min), 3 mL of the mixture was taken out and centrifuged. The supernatant (2 mL) was measured by a UV–Vis spectrometer to calculate the concentration of Congo red in solution. The adsorption kinetic curves were then obtained. For photodegradation experiments of Congo red, 40 mg of catalysts was dispersed in 50 mL Congo red (40 mg L−1) aqueous solution in a quartz tube. The above mixture was stirred for 30 min to reach the adsorption–desorption equilibrium in dark. Afterward, the suspensions were exposed to the UV-light irradiation with stirring. The irradiance value (1758–1734 W m−2) is tested by a radiometer FZ-A (Photoelectric Instrument Factory of Beijing Normal University, China) at the level of the photocatalytic process with 30 min preheating of mercury lamp. After different reaction times, 3 mL of suspension was extracted and centrifuged to get 2 mL supernatant without catalysts. The 2 mL supernatant was analyzed by the UV–Vis spectrophotometer to obtain the concentrations of the remaining Congo red. After measurement, the 2 mL supernatant and the centrifuged photocatalysts were resuspended by ultrasonication and fed back into the quartz tube. For reusing experiments, 40 mg of catalysts was dispersed in 50 mL Congo red (40 mg L−1) aqueous solution in a quartz tube. Under the same conditions with the above-mentioned photodegradation experiment, the solutions were irradiated under the UV light for 80 min. After irradiation, the solutions were centrifuged to recycle the catalysts. The 2 mL supernatant was extracted and analyzed by the UV–Vis spectrophotometer to obtain the concentrations of the remaining Congo red. The recycled photocatalysts were washed by water and ethanol for three times to remove extra Congo red. They were dried under vacuum oven at 60 °C overnight. The dried photocatalysts were weighed and mixed with Congo red solution with fixed concentration of catalyst to be 0.8 mg mL−1. The process was carried out for five continuous cycles to estimate their stability.
Characterizations
The sample morphologies were determined by SEM (Quanta 200 FEG; Netherlands) and TEM (H-7650 Hitachi; Japan). X-ray powder diffraction (XRD) was measured in the reflection mode (Cu Kα radiation) on a diffractometer (D/Max-RB, Japan) in the range of 20°–90°. UV–Vis spectra were obtained using a Cary 60 spectrophotometer (Agilent, US). The inductively coupled plasma optical emission spectroscopy (ICP-OES) was used to measure the Ag and Zn elements. The diffuse reflectance spectra were analyzed by Hitachi-4100 UV–Vis spectrophotometer (Japan) with an integrating sphere to record the diffuse reflectance spectra of the powder samples with BaSO4 as a reference.
Results and discussion
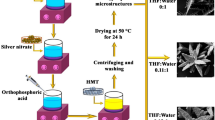
The pompon-like Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were prepared via a combination of precipitation reaction in the ethanol/water mixing reaction layer and room-temperature self-assembly as shown in Scheme 1. Through long-time ultrasonic treatment (2 h), the zinc sulfate monohydrate bulks were broken into small crystals in ethanol (Scheme 1a). Then, the diluted ammonium hydroxide solution (with water) was added and mixed with ethanol solution immediately. The zinc sulfate crystals were dissolved by water; meanwhile, the ethanol/water mixing layer containing zinc sulfate and ammonium hydroxide was formed (Scheme 1b). The two reactants in the mixing layer reacted to form the small particles of Zn(OH)1.68(SO4)0.16·0.58H2O, followed by their aggregating into microspheres (Scheme 1c). Due to the Ostwald ripening, the free nanoparticles spontaneously aggregate into 2D nanosheets on the Zn(OH)1.68(SO4)0.16·0.58H2O microspheres. The nanosheets were grown and assembled into 3D flower-like structures (Scheme 1d). By heating Zn(OH)1.68(SO4)0.16·0.58H2O microspheres at 800 °C for 8 h, the pompon-like ZnO microstructures were finally produced (Scheme 1e). It is a special non-template method using ethanol and water mixing layer to form microstructures.
The morphologies of the Zn(OH)1.68(SO4)0.16·0.58H2O microstructures and ZnO products were investigated by SEM as shown in Fig. 1. The Zn(OH)1.68(SO4)0.16·0.58H2O (Fig. 1a, b) and ZnO (Fig. 1d, e) microspheres both showed similar 3D pompon-like structure and similar size. Their diameters are 17.5 ± 5.6 and 19.1 ± 7.3 μm, respectively. In Fig. 1b, there are many regular nanosheets grown on the surface forming the special 3D pompon-like structure. The nanosheets of Zn(OH)1.68(SO4)0.16·0.58H2O microspheres (Fig. 1c) are smooth, but became porous after calcination (Fig. 1f). The thickness of Zn(OH)1.68(SO4)0.16·0.58H2O nanosheets is 2.3 ± 0.5 nm measured by AFM (Fig. S1).
The formation processes of Zn(OH)1.68(SO4)0.16·0.58H2O microstructures are shown in Fig. 2. The ZnSO4 crystals are marked by white arrows in Fig. 2. The transformation of ZnSO4 crystals to Zn(OH)1.68(SO4)0.16·0.58H2O hollow microstructures is clearly presented. The crystals of ZnSO4 still exist as shown in Fig. 2a after adding the diluted ammonium hydroxide solution into the crystal ethanol suspension at 1 s because the dissolution of crystals takes time. At 8 s (Fig. 2b), the ethanol/water mixing reaction layer can be observed clearly, where the precipitation of Zn(OH)1.68(SO4)0.16·0.58H2O took place to form microshells. And the thickness of one hollow shell is about 0.99 μm. The thickness was marked by red line. After the ammonium hydroxide solution was added, the crystals in ethanol are dissolved and become smaller (Fig. 2a–c) and finally disappeared at 17 s (Fig. 2d), while the thickness of the shell increased to 1.49 μm. The Zn(OH)1.68(SO4)0.16·0.58H2O hollow microstructures were successfully fabricated after 17 s using this method.
As shown in Fig. 3, the length of Zn(OH)1.68(SO4)0.16·0.58H2O nanosheets was controlled from 0.8 ± 0.4 to 4.4 ± 1.5 μm by room-temperature Ostwald ripening. The immature nanosheets in Fig. 3a were just like wrinkles on the surface. The fully developed flower-like structure was similar to the pompon mum flower (Fig. 3b). After 75 min, the nanosheets were fully developed. The dissolution and crystallization were in dynamic balance, and consequently the length was not changed (Fig. 3e) any more. The hollow structure of Zn(OH)1.68(SO4)0.16·0.58H2O microspheres is shown in Fig. S2. The hollow structure existed at the beginning of the ripening and was kept all the time. According to previous reports, Ostwald ripening (Li and Wang 2010; Zhu et al. 2009b) needs high temperature and long time with complex solvents; however, our attractive method can be operated at room temperature, with short time and without the introduction of surfactants.
The Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were further confirmed by element mapping based on energy-dispersive spectroscopy (EDS), where Zn, O and S are homogeneously distributed throughout the microspheres (Fig. 4a). As shown in Fig. 4b, clear and well-defined peaks of Zn, O and S can be observed in the EDS spectrum. From the EDS spectrum, the atomic fraction of microspheres can be further revealed (Fig. 4c), where the atomic ratio of Zn/O/S is 1:2.9:0.16. The possible molecular formula was proposed as Zn(OH)1.68(SO4)0.16·0.58H2O according to charge and matter conservation.
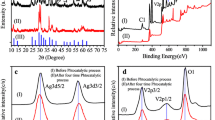
The TEM image (Fig. 5a) showed Ag–Ag2O nanoparticles deposited on ZnO surfaces. The nanosized (diameter of ~ 15 nm) Ag–Ag2O nanoparticles were homogeneously grown on the surface of ZnO. The TEM images of pure ZnO are shown in Fig. S3. It is clear that there are no nanoparticles on the surface, which confirmed that the nanoparticles on the surface are Ag–Ag2O. In Fig. S4, the TEM of ZnO/Ag–Ag2O microstructures after five cycling was given to show the stability. After five cycles, the Ag–Ag2O nanoparticles were still on the surface of ZnO, but the size and number were reduced. X-ray diffraction patterns of as-prepared samples are shown in Fig. 5b. Both ZnO sample and ZnO/Ag–Ag2O sample had same structure with standard ZnO (PDF 79-0206). The sharp peaks in the spectra demonstrate the high crystallinity of the products. The marked peaks from the enlarged XRD spectra were originated from the structures of Ag (2θ values of 38, 44 and 64) and Ag2O (2θ values of 33 and 38), which confirmed the particles are Ag–Ag2O nanoparticles. The ICP-OES experiment also proved the existence of Ag element in ZnO/Ag–Ag2O microstructures, with the ratio of Ag to Zn of 1:40. The diffuse reflectance spectra were measured to investigate the optical properties as shown in Fig. 5c, d. The absorption of ZnO is mainly less than 372 nm in the UV region and no significant absorbance can be observed in the visible light region from 400 to 800 nm. The Ag2O was reported with a band gap of 1.2 eV and showed strong absorption of both UV and visible light (Wang et al. 2011). Ag works well between the valence band and conduction band of ZnO, which may facilitate the light absorption capacity (Karunakaran et al. 2011; Zheng et al. 2008). Hence, with Ag–Ag2O modified on the surface of ZnO microstructures, the optical absorption of the composites can be greatly enhanced in both ultraviolet and visible light region which are related to their excellent photocatalytic activity. With the help of Ag–Ag2O nanoparticles and also the surface roughness increase, the absorption intensity of ZnO/Ag–Ag2O microstructures increased from 1.07 to 1.36. The roughness was measured by AFM and analyzed by Gwyddion software. By adding Ag–Ag2O nanoparticles, the value of Ra increased from 37.91 ± 2.54 to 71.19 ± 16.45 nm, and AFM images are shown in Fig. S5. The optical band gaps of ZnO and ZnO/Ag–Ag2O microstructures calculated by the Kubelka–Munk method were obtained to be 3.22 and 3.21 eV, respectively, as shown in Fig. 5d. It can be seen that the optical band gap of the ZnO microstructures slightly decreased by adding Ag–Ag2O nanoparticles, which was mainly due to the quantum size effects (Xue et al. 2008).
The TEM images of ZnO/Ag–Ag2O microstructures (a) and their XRD patterns (b). The image on the top of b is the enlarged XRD pattern in the dotted box for ZnO/Ag–Ag2O. UV–Vis diffuse reflectance spectra (c) and the corresponding plots to estimate the band gap values (d) of ZnO and ZnO/Ag–Ag2O microstructures by Kubelka–Munk equation
The adsorption and photocatalytic performances of ZnO/Ag–Ag2O microstructures under UV-light regions were investigated by degrading Congo red and methylene blue. Photodegradability-related curves are shown in Figs. 6 and 7. Figure 6a shows the adsorption capacity of pure ZnO and ZnO/Ag–Ag2O toward Congo red at room temperature. It can be seen that the adsorption is very fast at the beginning stage, and then it becomes slower and finally reaches equilibrium after 30 min. The maximum adsorption capacity is 15.7 and 31.5 mg g−1 for ZnO and ZnO/Ag–Ag2O, respectively. The adsorption kinetic curves according to the pseudo-second-order kinetic rate model (Eq. 1) are shown in Fig. 6b, where the pseudo-second-order rate constants were 0.02963 and 0.01356 g (min mg)−1 for ZnO and ZnO/Ag–Ag2O, respectively.
where k is the rate constant and qt (mg g−1) and qe (mg g−1) are the amount of dye sorbed at time t (min) and equilibrium state, respectively.
a Adsorption kinetics of pure ZnO and ZnO/Ag–Ag2O for Congo red; b the pseudo-second-order plot transformed from curve in (a); c photocatalytic degradation of Congo red with no photocatalysts (black curve), ZnO microspheres (red curve) and ZnO/Ag–Ag2O microstructures (blue curve). d The ln(C0/C) against time curves derived from (c); the insets are the images of Congo red solution after 80 min irradiation under different conditions. e The stability test of ZnO/Ag–Ag2O microstructures for Congo red
a Adsorption kinetics of pure ZnO and ZnO/Ag–Ag2O for methylene blue; the inset is a schematic diagram to explain the difference on the adsorption (electrostatic adsorption) for Congo red (CR) and methylene blue (MB); b the values of zeta potential for water control, ZnO and ZnO/Ag–Ag2O; c photocatalytic degradation of methylene blue with no photocatalysts (black curve), ZnO microspheres (red curve) and ZnO/Ag–Ag2O microstructures (blue curve). d The ln(C0/C) against time curves derived from (c). e The stability test of ZnO/Ag–Ag2O microstructures for methylene blue (decolorization efficiency and TOC removal). f Photocatalytic degradation of tetracycline with no photocatalysts (black curve) and ZnO/Ag–Ag2O microstructures (blue curve)
Due to adsorption process, 31.4% and 63.0% of total Congo red are removed for ZnO and ZnO/Ag–Ag2O, respectively. With the help of Ag–Ag2O composites, the adsorption ability increased. It can be explained by the effect of electrostatic adsorption shown in Fig. 7a, b. The ZnO/Ag–Ag2O (20.1 mV) held more positive charges than ZnO (13.2 mV); thus, the negatively charged Congo red are more liable to be adsorbed on ZnO/Ag–Ag2O. To study the photocatalysis degradation, the adsorption effect should be limited. At first, the system was conducted to equilibrium without illumination and adsorption–desorption equilibrium was reached after 30 min. After adsorption–desorption equilibrium, the removal of Congo red is mainly due to photocatalysis degradation. The characteristic peaks of Congo red at 496 nm decreased gradually without shifting and almost disappeared after 80 min irradiation. The degradation efficiency is calculated by Eq. 2:
where C is the concentration of the centrifuged solution after irradiation time t and C0 is the initial concentration of the organic pollution solution.
According to photocatalytic degradation curves in Fig. 6c, 83.2% and 96.3% degradation efficiency of Congo red were achieved by pure ZnO and ZnO/Ag–Ag2O after 80 min. The Ag–Ag2O composites and the increasing roughness of microstructures are the main factors to enhance degradation efficiency. The blank sample without catalysts showed only 29.5% degradation efficiency. The total organic carbon elimination efficiency of Congo red is shown in Fig. S6. 18.7% of 40 mg L−1 Congo red was thoroughly degraded into CO2 and H2O. The kinetic curve (Fig. 6d) was fitted using the pseudo-first-order kinetics equation, \({ \ln }\frac{{c_{0} }}{c} = kt\) where k was the first-order rate constant (min−1). The calculated k value and the square linear correlation coefficients (R2) are listed in Table 1. The k results clearly demonstrated that the ZnO/Ag–Ag2O microstructures had the better degradation rate constant of 0.04056 min−1, which is nearly twice as that of pure ZnO (0.02208 min−1). The stability of the catalyst was tested by five successive cycles for the degradation of Congo red under UV light, as shown in Fig. 6e. Degrading efficiency of five cycles were 96.3%, 94.8%, 95.6%, 95.7% and 96.8%, respectively, which confirmed excellent reusability of ZnO/Ag–Ag2O catalysts. The photocatalytic mineralization was presented through TOC removal. Only about 18.9% of total Congo red was completely removed in the five cycles. The performances of other photocatalysts for Congo red are listed in Table 2. It is noted that our catalyst displayed higher removal efficiency than other catalysts. The ZnO/Ag–Ag2O microstructures displayed great potential for Congo red photodegradation.
The degradation experiments for methylene blue were also presented in a similar way. The adsorption capacity of pure ZnO and ZnO/Ag–Ag2O toward methylene blue at room temperature is shown in Fig. 7a. Only 0.13 and 0.20 mg g−1 of methylene blue were adsorbed by ZnO and ZnO/Ag–Ag2O after 40 min. Compared with Congo red, the catalysts showed a poor adsorption capacity for methylene blue, which can be explained by the zeta potential properties of ZnO and ZnO/Ag–Ag2O. The zeta potential of water control, ZnO and ZnO/Ag–Ag2O are shown in Fig. 7b. The water samples were conducted (− 0.99 ± 0.92 mV) as a control group to make sure the accuracy. The ZnO and ZnO/Ag–Ag2O are positively charged due to their zeta potential of 13.2 mV and 20.1 mV, respectively, where the Ag–Ag2O nanoparticles contribute to the positive shift. The ZnO and ZnO/Ag–Ag2O catalysts are available to adsorb the negatively charged dye, while the methylene blue holds positive charges and Congo red has negative charges according to the molecular formula in the insets of Fig. 7a. The photocatalytic degradation curves are shown in Fig. 7c. 78.2% and 95.6% degradation efficiency of methylene blue were achieved by pure ZnO and ZnO/Ag–Ag2O after 40 min. Finally, degradation efficiency with pure ZnO and ZnO/Ag–Ag2O was 95.3% and 99.5% at 80 min, respectively, while the blank sample without catalysts showed only 1.0% degradation efficiency. The kinetic curves (Fig. 7d) were fitted using the pseudo-first-order kinetics equation, where the calculated k values and R2 coefficients are listed in Table 3. The k and degradation efficiency clearly demonstrated that the ZnO/Ag–Ag2O microstructures had the better degradation ability than that of pure ZnO. The reusability of ZnO/Ag–Ag2O catalysts for methylene blue was shown by the recycling experiment in Fig. 7e. The removal efficiency of the dyes with five cycles was all more than 97.5% and the removal efficiency of total organic carbon was nearly 95.1%, which confirmed excellent catalytic stability. Compared with other photocatalysts as shown in Table 4, ZnO/Ag–Ag2O microstructures show excellent degradation in both decolorization efficiency and TOC removal. The ZnO/Ag–Ag2O microstructures exhibited great potential for methylene blue photodegradation. Tetracycline was also chosen as the degradation target with an initial concentration of 10 mg L−1. The suspension is irradiated under UV light for 80 min after stirring for 30 min in a dark environment. The results are shown in Fig. 7f. The photodegradation efficiency of tetracycline is 97.1% after 80 min irradiation. These results clearly demonstrated that ZnO/Ag–Ag2O microstructures can be used as an efficient photocatalyst for the organic wastewater treatment.
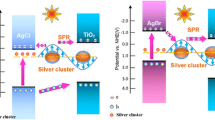
Under UV irradiation, both Ag2O and ZnO can be simultaneously excited to form electron–hole pairs. Due to the different band gap energy (Wu et al. 2012; Ma et al. 2014), the photogenerated electrons transferred from ZnO to Ag2O, while holes diffused from Ag2O to ZnO. With Ag doping, the electrons in Ag2O can arrive at the interface easily for the short mean free paths and help the charge separation (Xue et al. 2008; Umukoro et al. 2016). Due to the fast charge-transfer effect of Ag (Zheng et al. 2007), the photogenerated electrons will store on the surface of Ag nanoparticles producing superoxide oxygen radical (·O2−), as shown in Fig. 8. Meanwhile the holes on the ZnO reacted with water to form hydroxyl radical (·OH) (Zhao et al. 2017; Gu et al. 2009). These radicals are the oxidants to decompose the organic dyes (Liu et al. 2013; Konstantinou and Albanis 2004).
Conclusions
ZnO/Ag–Ag2O microstructures were synthesized for Congo red photocatalytic degradation. It is the first time that the Zn(OH)1.68(SO4)0.16·0.58H2O microspheres were fabricated by combining dynamic dissolving/precipitation reaction in ethanol/water mixing layer and particle self-assembly. The ripening of Zn(OH)1.68(SO4)0.16·0.58H2O microspheres happened at room temperature and within shorter time period compared with conventional ripening process. Although the ZnO microspheres can be used to degrade Congo red directly, the addition of Ag–Ag2O nanoparticles enables double reaction rate and enhanced the degradation efficiency by 13.1% from 83.2 and 96.3%. Compared with previously reported ZnO/Ag photocatalysts (Guy and Ozacar 2016) with 81.6% removal for Congo red, our result enhanced degradation efficiency by 14.7%. The ZnO/Ag–Ag2O microstructures hold great potential for organic pollutant photodegradation with high removal efficiency.
Abbreviations
- SEM:
-
Scanning electron microscopy
- UV:
-
Ultraviolet
- XRD:
-
X-ray powder diffraction
- R 2 :
-
Square linear correlation coefficients
- C :
-
Remaining concentration of organic pollutants
- C 0 :
-
Initial concentration of organic pollutants
References
Chen H, Zhao L, Wang GH, He X, Wang XT, Fang W, Du X (2017) Direct growth of ZnO nanorods on biogenic hierarchical rice husk SiO2 and their application to dye degradation. Clean Technol Environ 19:1335–1345
Ciciliati MA, Silva MF, Fernandes DM, de Melo MAC, Hechenleitner AAW, Pineda EAG (2015) Fe-doped ZnO nanoparticles: synthesis by a modified sol–gel method and characterization. Mater Lett 159:84–86
Fu YS, Huang T, Zhang LL, Zhu JW, Wang X (2015) Ag/g–C3N4 catalyst with superior catalytic performance for the degradation of dyes: a borohydride-generated superoxide radical approach. Nanoscale 7:13723–13733
Gu CD, Cheng C, Huang HY, Wong TL, Wang N, Zhang TY (2009) Growth and photocatalytic activity of dendrite-like ZnO@Ag heterostructure nanocrystals. Cryst Growth Design 9:3278–3285
Guo SY, Zhao TJ, Jin ZQ, Wan XM, Wang PG, Shang J, Han S (2015) Self-assembly synthesis of precious-metal-free 3D ZnO nano/micro spheres with excellent photocatalytic hydrogen production from solar water splitting. J Power Sources 293:17–22
Guruvammal D, Selvaraj S, Sundar SM (2016) Effect of Ni-doping on the structural, optical and magnetic properties of ZnO nanoparticles by solvothermal method. J Alloys Compd 682:850–855
Guy N, Ozacar M (2016) The influence of noble metals on photocatalytic activity of ZnO for Congo red degradation. Int J Hydrogen Energy 41:20100–20112
Ismail AA, Faisal M, Al-Haddad A (2018) Mesoporous WO3-graphene photocatalyst for photocatalytic degradation of methylene blue dye under visible light illumination. J Environ Sci China 66:328–337
Jesudoss SK, Vijaya JJ, Selvam NCS, Kombaiah K, Sivachidambaram M, Adinaveen T, Kennedy LJ (2016) Effects of Ba doping on structural, morphological, optical, and photocatalytic properties of self-assembled ZnO nanospheres. Clean Technol Environ 18:729–741
Kadirova ZC, Hojamberdiev M, Katsumata KI, Isobe T, Matsushita N, Nakajima A, Okada K (2017) Fe2O3-loaded activated carbon fiber/polymer materials and their photocatalytic activity for methylene blue mineralization by combined heterogeneous-homogeneous photocatalytic processes. Appl Surf Sci 402:444–455
Karunakaran C, Rajeswari V, Gomathisankar P (2011) Enhanced photocatalytic and antibacterial activities of sol–gel synthesized ZnO and Ag–ZnO. Mat Sci Semicond Proc 14:133–138
Konstantinou IK, Albanis TA (2004) TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations—a review. Appl Catal B Environ 49:1–14
Lee SS, Bai HW, Liu ZY, Sun DD (2013) Novel-structured electrospun TiO2/CuO composite nanofibers for high efficient photocatalytic cogeneration of clean water and energy from dye wastewater. Water Res 47:4059–4073
Li BX, Wang YF (2010) Facile synthesis and enhanced photocatalytic performance of flower-like ZnO hierarchical microstructures. J Phys Chem C 114:890–896
Liu SY, Cai Y, Cai XY, Li H, Zhang F, Mu QY, Wang YJ, Wang YD (2013) Catalytic photodegradation of Congo red in aqueous solution by Ln(OH)3 (Ln = Nd, Sm, Eu, Gd, Tb, and Dy) nanorods. Appl Catal a General 453:45–53
Ma SS, Xue JJ, Zhou YM, Zhang ZW (2014) Photochemical synthesis of ZnO/Ag2O heterostructures with enhanced ultraviolet and visible photocatalytic activity. J Mater Chem A 2:7272–7280
Miranda LDL, Bellato CR, Milagres JL, Moura LG, Mounteer AH, de Almeida MF (2015) Hydrotalcite-TiO2 magnetic iron oxide intercalated with the anionic surfactant dodecylsulfate in the photocatalytic degradation of methylene blue dye. J Environ Manag 156:225–235
Mohanta O, Singhbabu YN, Giri SK, Dadhich D, Das NN, Sahu RK (2013) Degradation of Congo red pollutants using microwave derived SrFe12O19: an efficient magnetic photocatalyst under visible light. J Alloys Compd 564:78–83
Pouretedal HR, Beigy H, Keshavarz MH (2010) Bleaching of Congo red in the presence of ZnS nanoparticles, with dopant of Co2+ ion, as photocatalyst under UV and sunlight irradiations. Environ Technol 31:1183–1190
Sampaio MJ, Lima MJ, Baptista DL, Silva AMT, Silva CG, Faria JL (2017) Ag-loaded ZnO materials for photocatalytic water treatment. Chem Eng J 318:95–102
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648–8649
Sun JH, Wang YK, Sun RX, Dong SY (2009) Photodegradation of azo dye Congo Red from aqueous solution by the WO3–TiO2/activated carbon (AC) photocatalyst under the UV irradiation. Mater Chem Phys 115:303–308
Trandafilovic LV, Jovanovic DJ, Zhang X, Ptasinska S, Dramicanin MD (2017) Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO: Eu nanoparticles. Appl Catal B Environ 203:740–752
Umukoro EH, Peleyeju MG, Ngila JC, Arotiba OA (2016) Photocatalytic degradation of acid blue 74 in water using Ag–Ag2O–ZnO nanostuctures anchored on graphene oxide. Solid State Sci 51:66–73
Wang XF, Li SF, Yu HG, Yu JG, Liu SW (2011) Ag2O as a new visible-light photocatalyst: self-stability and high photocatalytic activity. Chem Eur J 17:7777–7780
Wang H, Liang LF, Cheng XJ, Luo YM, Sun S (2018) Facile fabrication of porous ZnS and ZnO films by coaxial electrospinning for highly efficient photodegradation of organic dyes. Photochem Photobiol 94:17–26
Wu M, Yan JM, Zhao M, Jiang Q (2012) Facile synthesis of an Ag2O–ZnO nanohybrid and its high photocatalytic activity. ChemPlusChem 77:931–935
Xue H, Xu XL, Chen Y, Zhang GH, Ma SY (2008) Influence of Ag-doping on the optical properties of ZnO films. Appl Surf Sci 255:1806–1810
Yan F, Liu Y, Cai X, Wu X (2016) Facile synthesis of Ag2O nanoparticles decorated ZnO assembles with excellent photocatalytic performances. J Nanosci Nanotechnol 16:8538–8543
Zhang YA, Xu JQ, Xu PC, Zhu YH, Chen XD, Yu WJ (2010) Decoration of ZnO nanowires with Pt nanoparticles and their improved gas sensing and photocatalytic performance. Nanotechnology:21
Zhao XL, Su YC, Qi XD, Han XJ (2017) A facile method to prepare novel Ag2O/Ag2CO3 three-dimensional hollow hierarchical structures and their water purification function. ACS Sustain Chem Eng 5:6148–6158
Zheng YH, Zheng LR, Zhan YY, Lin XY, Zheng Q, Wei KM (2007) Ag/ZnO heterostructure nanocrystals: synthesis, characterization, and photocatalysis. Inorg Chem 46:6980–6986
Zheng YH, Chen CQ, Zhan YY, Lin XY, Zheng Q, Wei KM, Zhu JF (2008) Photocatalytic activity of Ag/ZnO heterostructure nanocatalyst: correlation between structure and property. J Phys Chem C 112:10773–10777
Zhu HY, Jiang R, Xiao L, Chang YH, Guan YJ, Li XD, Zeng GM (2009a) Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J Hazard Mater 169:933–940
Zhu LP, Liao GH, Yang Y, Xiao HM, Wang JF, Fu SY (2009b) Self-assembled 3D flower-like hierarchical beta-Ni(OH)2 hollow architectures and their in situ thermal conversion to NiO. Nanoscale Res Lett 4:550–557
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFC0401104), HIT Environment and Ecology Innovation Special Funds (HSCJ201617), the National Natural Science Foundation of China (Grant Nos. 21773050, 21528501), and State Key Laboratory of Urban Water Resource and Environment (Harbin Institute of Technology) (No. 2017DX05).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Su, Y., Zhao, X., Bi, Y. et al. ZnO/Ag–Ag2O microstructures for high-performance photocatalytic degradation of organic pollutants. Clean Techn Environ Policy 21, 367–378 (2019). https://doi.org/10.1007/s10098-018-1641-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-018-1641-0