Abstract
An experimental investigation was conducted on the ultrasound-assisted oxidative desulfurization of non-hydrotreated kerosene. The influences of various operating parameters including oxidant-to-sulfur molar ratio (n o/n s), formic acid-to-sulfur molar ratio (n acid/n s), the ultrasound power per fuel oil volume, sonication time, and temperature on the sulfur removal of kerosene have been investigated. Response surface methodology based on Box–Behnken design has been employed. 95.46 % sulfur removal of kerosene has been achieved in the sonication time of 10.5 min under the optimal oxidation conditions (i.e., n o/n s = 15.02, n acid/n s = 107.8, and the ultrasound power per fuel oil volume of 7.6 W/mL) followed by liquid–liquid extraction. More than 90 % sulfur removal of kerosene has been achieved in about 3.5 min of sonication followed by the liquid–liquid extraction. 70.2 % sulfur removal of kerosene has been achieved in the same operating conditions after 3.5 min of oxidation followed by the liquid–liquid extraction in the absence of ultrasound irradiation. The effects of the number of extraction stages on the desulfurization and recovery of kerosene have been also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The presence of sulfur-containing compounds in the middle distillate fuels leads to the emission of SOx to the atmosphere and acid rain (Srivastava 2012). Nowadays, very stringent worldwide regulations on the sulfur content of fuel oils have been legislated due to the environmental concerns (Al-Lal et al. 2015). Kerosene is a complex mixture of alkanes, aromatics, and cycloalkanes. Kerosene is widely used in the industries, especially as aircraft fuel (Dagaut et al. 2006). At present, the conventional desulfurization process of middle distillate fuels is hydrodesulfurization (HDS). It should be noted that HDS has limited capability for the removal of thiophenic compounds which are the main sulfur-containing compounds of the kerosene and diesel fuels. To meet new sulfur standards using HDS process, operation at high pressure and temperature, application of more active catalysts, and high hydrogen consumption are inevitable (Calcio Gaudino et al. 2014). Several HDS alternative or complementary processes such as oxidative desulfurization (ODS) (Campos-Martin et al. 2010), selective adsorption (Yang et al. 2003), and bio-desulfurization (Villasenor et al. 2004) have been proposed. Comparing these different HDS alternative processes, the ODS process seems more promising because of its several advantages relative to the HDS process. For instance, the ODS process can be carried out in the liquid phase and under the mild pressure and temperature conditions. In addition, the reactivity of HDS refractory sulfur-containing compounds (e.g., thiophenic compounds and its alkylated derivatives) is high toward oxidation.
In the ODS process, the sulfur-containing compounds are oxidized to their corresponding sulfoxides and sulfones. Then, these polar oxidized products can be removed readily by a number of conventional separation processes such as adsorption, and solvent extraction.
Different oxidants and catalysts have been examined in the ODS process (Sobati et al. 2010b). Various oxidation systems such as hydrogen peroxide/MoO3/Al2O3 catalyst (Akbari et al. 2014), Hydrogen peroxide/phosphotungstic acid supported on the activated carbon (Liu et al. 2014), hydrogen peroxide/acetic acid (Margeta et al. 2016), hydrogen peroxide/formic acid (Sobati et al. 2010c), tungstophosphoric acid/tetraoctylammonium bromide as a phase-transfer agent (PTA)/ hydrogen peroxide (Kadijani et al. 2015) have been studied.
Hydrogen peroxide and formic acid oxidation system, which produces in situ peroxyformic acid has several advantages such as commercial availability of formic acid as the catalyst, simplicity of the oxidation system in comparison with other oxidation systems, etc (Sobati et al. 2010c).
It should be noted that the nature of this system is biphasic (i.e., aqueous phase, and hydrocarbon phase). In other words, the overall rate of the oxidation of sulfur-containing compounds can be influenced by both physical and chemical phenomena (De Filippis et al. 2010).
In the present oxidation system, effective mixing between heterogeneous phases (i.e., the aqueous phase, including hydrogen peroxide/formic acid and the hydrocarbon phase) is one of the main challenges in our effort to enhance the oxidation rate of the sulfur-containing compounds. Ultrasonic irradiation is a process of emission of compressed waves with frequencies above the range of human hearing (Hajamini et al. 2016). The application of ultrasound irradiation can intensify the chemical reactions due to creation of very fine emulsion in the organic and aqueous medium (Nunes et al. 2014). The combined use of ultrasound irradiation, the oxidizer, and the catalyst followed by a liquid–liquid extraction or an adsorption process to remove sulfur-containing compounds from the fuel is called ultrasound-assisted oxidative desulfurization (UAOD) (Choi et al. 2015).
UAOD can enhance mass transfer and inherent reaction kinetics of the oxidative desulfurization process due to the creation of cavities in the liquid fuels (Bhasarkar et al. 2015a). Cavities produced by ultrasound irradiation increase the liquid–liquid interfacial area for the viscous films containing gas-filled bubbles, which in turn leads to the higher reaction rate. Formation of the fine emulsion between immiscible phases is considered as the physical effect of ultrasonic cavitation, and the production of radicals through transient collapse of cavitation bubbles can be considered as the chemical effect of ultrasonic cavitation (Kuppa and Moholkar 2010).
The UAOD performance can be influenced by the nature of hydrocarbon feedstock and the distillation range (Ismagilov et al. 2011).
Most of the UAOD studies were focused on the diesel fuels and model fuels with single-specific sulfur-containing compounds such as dibenzothiophene (DBT). There are few reports available regarding the oxidative desulfurization of kerosene and jet fuels. Table 1 shows a number of previous studies considering sulfur removal of the kerosene feedstocks using different oxidative desulfurization processes.
One of the main limitations of the previous works is the high oxidation time. For example, according to Dehkordi et al. (Dehkordi et al. 2009a), at least 30 min of oxidation time is required to achieve 87 % sulfur removal. One of the main targets of the present study is to reduce the required reaction time using ultrasound irradiation in a simple and inexpensive oxidation system.
Bhasarkar et al. have applied a low-power 70-W ultrasonic bath device for the oxidation of model sulfur-containing compounds using hydrogen peroxide, fenton–peracetic acid oxidation system. Their reported sulfur conversion after 1 h was 30 % (Bhasarkar et al. 2013). They also applied the same device for the oxidation of model sulfur-containing compounds using hydrogen peroxide, formic acid, and phase-transfer agent (PTA) oxidation system. Their reported sulfur conversion after 1 h was 47 % (Bhasarkar et al. 2015a). In their proposed oxidation system, the PTA facilitates the interphase transport of the oxidizing agent and leads to an increase in the conversion of sulfur-containing compound (Bhasarkar et al. 2015b). The aim of the present study is to remove the expensive phase-transfer agents from the oxidation system through the application of high-power direct probe ultrasound system.
In the present work, the effects of formic acid and hydrogen peroxide quantities and the ultrasound power per fuel volume on the sulfur removal of kerosene fuel are investigated using an inexpensive system in the absence of phase-transfer agent. In addition, the effect of sonication time is studied in details, and the performance of the UAOD is compared with the conventional oxidative desulfurization (ODS).
Response surface methodology (RSM)—Box–Behnken design—is applied in order to study the influences of the different operating variables on the sulfur removal of kerosene. In the recent years, RSM has been applied to reduce the required experimental data in order to achieve the best operating conditions for a desired response in numerous chemical, and biochemical processes (Abdullah et al. 2014).
For instance, RSM-Box–Behnken design has been employed for the investigation of the UAOD of model fuels using hydrogen peroxide/ferrate/PTA (Choi et al. 2014), and hydrogen peroxide/phosphotungstic acid/PTA (Lu et al. 2014). The RSM-Box–Behnken design has been also employed for the investigation of the UAOD of real diesel fuel using H2O2/tungstophosphoric acid/tetra octylammonium bromide (Kadijani et al. 2015).
The effect of the number of extraction stages on the sulfur removal of kerosene is also investigated. As one of the main practical problems of the ODS process, a considerable hydrocarbon loss is incurred in the extraction step due to the simultaneous extractions of the hydrocarbon compounds present in the kerosene feedstock (Sobati et al. 2010b). Therefore, a simple method for the reduction of hydrocarbon loss is also introduced.
Experimental
Chemicals
The chemicals used in the this study such as formic acid (99 wt%), hydrogen peroxide (30 wt%) were obtained from Merck Co. (Germany). Acetonitrile (99 wt%) was obtained from Duksan (korea). All of the mentioned chemicals were of analytic grades and were used without further purification. Non-hydrotreated kerosene as a feedstock with total sulfur content of 2490 ppmw was obtained from Tehran refinery (Tehran, Iran). The specifications of kerosene feedstock are summarized in Table 2.
Method of the analyses
The total sulfur content of the kerosene samples were determined by Rigaku NEXQC+ (Rigaku, Japan). NEXQC+ has the total sulfur detection range of 0–5 wt%, with a lower detection limit of 25 ppm, and the test method is based on the wavelength dispersive X-ray fluorescence technique according to the ASTM D-4294.
Agilent 6890 series gas chromatograph system coupled with Agilent 5973 network mass selective detector and capillary column DB-1 ms was applied to detect sulfur-containing compounds in the kerosene feedstock qualitatively. The injector temperature was set to 250 °C. The initial temperature was set to 50 °C for 5 min, and then the temperature was increased to 275 °C with temperature increasing at a rate of 5 °C/min and then kept constant at 275 °C for 10 min. A Chrompack CP 9000 gas chromatograph equipped with a flame photometric detector (GC-FPD) was used to analyze the sulfur-containing compounds in the untreated and treated kerosene. The chromatography column was fused-silica capillary (CP-Sil 8 CB, from Supelco Company). The injector was set at 270 °C and the detector at 300 °C. The nitrogen with a constant rate of 0.5 mL/min was used as the carrier gas. General properties of the kerosene feedstock such as paraffin, naphthene, olefin, aromatic, water contents, and total acid number were determined according to the corresponding ASTM methods.
Experimental setup and procedures
In each UAOD run, 50 mL of kerosene feedstock with a total sulfur content of 2490 ppmw was introduced into a 75-mL jacket-type reactor with an internal diameter of 5 cm. The experimental setup was also equipped with a condenser and an electronic thermometer (Fig. 1). The reactor temperature was kept constant during ultrasound irradiation by passing water through the reactor jacket.
Experimental setup of the ultrasound-assisted oxidative desulfurization (UAOD). 1 20-kHz ultrasound generator; 2 transducer; 3 sealing joint; 4 probe; 5 reactor; 6 reactor jacket; 7 condenser; 8 formic acid, hydrogen peroxide entering connection; 9 temperature sensor; 10 cold water circulation pumps; 11 constant-temperature water bath; 12 extractor flask; 13 magnetic stirrer; 14 separatory funnel and its stand; 15 total sulfur analyzer
Titanium ultrasonic probe (12 mm diameter) of 20 kHz frequency (UTD 400, Ultrasound Technology Development Company, Iran) was used in the UAOD system. The reaction mixture was directly irradiated by the ultrasound probe in such a way that 1 cm of the ultrasound probe was immersed into the reaction mixture. The power of ultrasound irradiation was regulated according to the experimental design. The reaction temperature was set to the predefined value by passing water to the jacket of the reactor. The desired amount of formic acid was added to the reactor. Afterward, an appropriate amount of hydrogen peroxide was injected into the reaction mixture, and the time was recorded as the onset of the oxidation reaction.
After passing the pre-defined sonication time, the aqueous and hydrocarbon phases in the reaction mixture were separated by a 100-mL separatory funnel. Then, the hydrocarbon phase (i.e., oxidized kerosene) was washed by 150 mL of distillated water, and the aqueous and hydrocarbon phases were separated by a 250-mL separatory funnel after 10 min. In the next step, the oxidized kerosene was extracted using acetonitrile according to the solvent-to-kerosene volume ratio of 1 at ambient temperature. In each extraction run, the kerosene and solvent were mixed under the stirring rate of 700 rpm for 30 min at the room temperature. The dispersion was formed and then separated into two distinct hydrocarbon and aqueous phases in a 100-mL separatory funnel after 20 min. In order to remove any entrained solvent from the treated kerosene, the treated hydrocarbon phase was washed by 50 mL of distillated water, and the aqueous and hydrocarbon phases were separated by a 100-mL separatory funnel after 1 min. Finally, about 30 mL of the treated kerosene sample was withdrawn for the total sulfur analysis. The hydrocarbon loss in each extraction step was evaluated, as well. It should be noted that several extraction steps were applied in some experiments in order to study the effects of the number of extraction stages on the sulfur removal.
Design of the experiments
Response surface methodology (RSM) is an appropriate tool for modeling, analysis, and evaluation of the interaction effects of different operating variables. In the present study, the Box–Behnken design (BBD) of RSM was applied.
The effect of three oxidation operating variables including oxidant-to-sulfur molar ratio (n o/n s), acid-to-sulfur molar ratio (n acid/n s), and the ultrasound power per kerosene volume (W/mL) on the response (i.e., the sulfur removal of kerosene) was studied. It should be noted that the sonication time of 10.5 min and temperature of 50 °C were set for the experimental design. It should be added that the appropriate range for different operating parameters was selected based on a set of preliminary oxidation experiments. Table 3 represents the experimental ranges and factor levels of the UAOD process.
According to the Box–Behnken design (BBD), 17 experimental runs were required, which included five replicates of the central run.
To correlate the sulfur removal of kerosene in the UAOD process, a quadratic polynomial equation was developed as a function of independent variables and their interactions. The general form of the correlation is as follows:
where Y is the predicted response for the process (i.e., the sulfur removal of kerosene), β 0 is the intercept coefficient (offset), β i are the parameters of the linear terms, β ii are the parameters of the quadratic terms, and β ij are the parameters of the interaction terms. X i and X j are the independent variables, and ε is the unanticipated error.
Results and discussions
According to the literature, the main sulfur-containing compounds of kerosene feedstock are benzothiophene and its alkylated derivatives (Sobati et al. 2010c). The GC-MS analysis results of the present work indicate that the kerosene contains benzothiophene and its alkylated derivatives such as 2,5,7-trimethyl benzothiophene, 4,5,6,7-tetramethyl benzothiophene, and also 2-ethyl-4-methyl thiophene.
Statistical analysis
The design of the experiments, the experimental results, and the predicted response (i.e., the sulfur removal of kerosene) are given in Table 4.
The RSM proposed a quadratic correlation that relates the sulfur removal to the independent variables (Table 5).
The sulfur removal is the response, and A, B, and C are the coded forms of the studied parameters. The sign and the value of the coefficient of each correlation term show the increasing and decreasing effects of the parameters on the response. According to the proposed correlation, the relative importance of the independent factors is as follows: B (i.e., formic acid-to-sulfur molar ratio) with the value of +9.32 for the coefficient; C (i.e., the ultrasound power per kerosene volume) with the value of +5.26 for the coefficient; and A (i.e., oxidant-to-sulfur molar ratio). The maximum increasing effect of the dependent factor is related to the interaction between A and B parameters with the value of +0.49 for the coefficient.
Table 6 shows the results of ANOVA test and the statistical parameters of the proposed quadratic correlation.
As can be observed, the model P value is lower than 0.0001, which indicates that the model terms are significant. It should be noted that a P value lower than 0.05 specifies the significant factors of the model. Moreover, the terms in the model have a significant effect on the response due to the large F-value (i.e., 128.01) (Montgomery and Runger 2010). It can be inferred from the coefficient of determination (R 2 = 0.994) that the correlation fits the data very well. In other words, 99.4 % of the total variation in the response was justified by the proposed quadratic correlation. The adequate precision of this model is 38.682, which shows the noise ratio of the model is placed in the desirable range. The experimental versus the predicted values of the sulfur removal is shown in Fig. 2.
According to this figure and the results of the ANOVA analysis, the proposed correlation is valid. A, B, C, AC, B2, C2 parameters are the most significant terms of the proposed correlation.
Interaction between different operating parameters
The response surface plots of the interactions between the independent parameters (i.e., n o/n s, n acid/n s, and the ultrasound power per kerosene volume) and the sulfur removal are shown in Fig. 3.
Response surface plots of the sulfur removal (%) as a function of (a) n o/n s and n acid/n s, (b) n o/n s and ultrasound power per kerosene volume, and (c) n acid/n s and ultrasound power per kerosene volume. Other oxidation conditions: T = 50 °C, and sonication time = 10.5 min; extraction conditions: ambient temperature; solvent: acetonitrile with solvent-to-kerosene volume ratio = 1; no. of extraction stages = 1
Figure 3a relates the oxidant-to-sulfur molar ratio (n o/n s) and the acid-to-sulfur molar ratio (n acid/n s) with the sulfur removal. As can be observed, an increase in (n o/n s) at the low values of (n acid/n s) leads to a slight decrease in the sulfur removal. This effect can be attributed to the reduction of the formic acid concentration in the aqueous phase, which in turn can decrease the peroxyformic acid concentration.
The results also indicate increasing (n acid/n s) up to 100 at different values of (n o/n s) increases the sulfur removal. However, no considerable improvement in the sulfur removal is observed by further increase in (n acid/n s) beyond this limit (zone 1). An observed increase in the sulfur removal in zone 1 can be attributed to the increase in the peroxyformic acid concentration as the main oxidizing agent in the present oxidation system (Dehkordi et al. 2009a). Although it seems that there is an equilibrium concentration for peroxyformic acid in the oxidation system, the sulfur removal is not changed by further increase in (n acid/n s) (zone 2).
The kinetics of peroxyformic acid formation has been reported in the literature (De Filippis et al. 2009). According to the literature, peroxyformic acid is produced by a reversible reaction of formic acid and hydrogen peroxide in the aqueous phase (De Filippis et al. 2009):
The amounts of hydrogen peroxide and formic acid should be selected in such a way to maximize the peroxyformic acid concentration in the aqueous phase in order to increase the sulfur removal.
The higher values of formic acid concentration lead to the lower pH values in the aqueous phase. The presence of proton sources results in the improvement of the oxidative desulfurization performance (Gonzalez et al. 2012).
The peroxyformic acid is also decomposed to formic acid, CO2, O2, and water (reactions 3 and 4). In other words, the peroxyformic acid concentration in the aqueous media is determined by taking into account the all reactions including the formation and decomposition of peroxyfromic acid. Hydrogen peroxide as an important reactant in reaction 1 also decomposes to water and oxygen (reaction 5) (De Filippis et al. 2009).
The observed trend of sulfur removal versus (n acid/n s) in zone 2 can be attributed to attainment to the equilibrium concentrations of formic acid, hydrogen peroxide, and peroxyformic acid in the reactions mixture.
Figure 3b relates the oxidant-to-sulfur molar ratio (n o/n s) and the ultrasound power per kerosene volume with the sulfur removal. As can be observed, increasing the (n o/n s) and the ultrasound power per kerosene volume up to the saddle point of the surface plot leads to higher values of the sulfur removal (zone 1). The observed trend in this zone can be attributed to the effective cavitation created by the ultrasonic irradiation and the subsequent physical effects such as ultra-micromixing and emulsification, which in turn leads to the enhancement of the overall mass transfer and the oxidation reaction rate (Bhasarkar et al. 2015a). However, no considerable improvement in the sulfur removal is observed in case of using higher ultrasound power per kerosene volume and the values of (n o/n s) larger than 7 and 25, respectively (zone 2). The observed trend in zone 2 can be explained as follows:
A sharp increase in the ultrasound power per kerosene volume beyond a certain limit leads to the generation of the large bubbles around the probe tip, which in turn lower the transferred frequency, and leads to a subsequent decrease in the micromixing effect (Žnidarčič et al. 2015). In addition, an increase in (n o/n s) leads to an increase in the volume of the aqueous phase and also production of vapor bubble instead of gas bubbles, which in turn limits the acoustic mixing efficiency.
Figure 3c relates the oxidant-to-sulfur molar ratio (n acid/n s) and the ultrasound power per kerosene volume with the sulfur removal. This figure indicates increasing the (n acid/n s) and the ultrasound power per kerosene volume up to a certain limit (i.e., about 7 W/mL) causes enhancement of the sulfur removal. This trend can be attributed to the increase in the peroxyformic acid concentration in the reaction media and intense micromixing due to the acoustic cavitation as a result of the ultrasound irradiation (Bolla et al. 2012). However, there is no considerable improvement in the sulfur removal by raising the ultrasound power per kerosene volume larger than 7 and the value of (n o/n s) larger than 100 due to similar reasons as mentioned above.
Nevertheless, Fig. 3a, c displays continuous increase in the sulfur removal with the increasing (n acid/n s). This observation can be attributed to the additional role of formic acid as the extraction solvent of the oxidized sulfur-containing compounds (Sobati et al. 2010b). In other words, formic acid as a polar compound can extract some of the oxidized sulfur-containing compounds into the aqueous phase of the oxidation system, simultaneously.
According to De Filippis et al. for hydrogen peroxide–formic acid oxidation system, the oxidation reactions of the sulfur-containing compounds are mainly carried out in the organic phase (De Filippis et al. 2011). The extra formic acid as a polar compound can facilitate the in situ extraction of the polar oxidation products (i.e., sulfoxides, and sulfones) into the aqueous phase of the oxidation system. The in situ partial extraction of sulfoxide and sulfone products leads to a decline of the total sulfur content of the oxidized kerosene and an increase in the final sulfur removal after oxidation followed by the liquid–liquid extraction.
The best UAOD operating parameters
One of the aims of this study was to find a combination of independent variables (i.e., nO/n s, n acid/n s, and the ultrasound power per kerosene volume) in such a way to achieve the maximum sulfur removal. According to the implemented experiments, the RSM optimization suggested different combinations of the variables to achieve over 95 % sulfur removal of kerosene. In this regard, an optimization run with 97.16 % sulfur removal was selected. This sulfur removal efficiency can be achieved under the following conditions: ((n o/n s) of 15.02, (n acid/n s) of 107.8, the ultrasound power per kerosene of 7.6) in the ultrasound-assisted oxidation followed by a single extraction stage using acetonitrile. Three replicate experiments were conducted under the proposed optimal conditions. As can be observed in Table 7, the average experimental sulfur removal (i.e., 95.37 %) is in close agreement with the predicted value (i.e., 97.16 %). In other words, the validity of the proposed model is confirmed again.
The effects of sonication time and temperature on the sulfur removal
Figure 4 shows the effect of sonication time on the sulfur removal of kerosene under the optimal conditions.
The sulfur removal of kerosene versus oxidation time. Oxidation conditions: UAOD: n o/n s = 15.02, n acid/n s = 107.8, T = 50 °C, and ultrasound power per fuel oil volume = 7.6 W/mL. Conventional ODS: n o/n s = 15.02, n acid/n s = 107.8, T = 50 °C, under magnetic stirring (rotation speed = 500 rpm). Extraction conditions: ambient temperature; solvent: acetonitrile with solvent-to-kerosene volume ratio = 1; no. of extraction stages = 1
This figure shows if the sonication time takes longer than 10.5 min no more considerable improvement in the sulfur removal can be achieved. As an important improvement, over 90 % of the sulfur removal could be achieved within the first 3.5 min of sonication time.
Figure 4 also demonstrates a comparison between UAOD and the conventional ODS under the identical operating conditions. In the conventional ODS experiments, magnetic stirring with the rotation speed of 500 rpm was used. As can be observed, considerable improvement in the sulfur removal was observed in case of UAOD in comparison with the conventional ODS. For example, only 70.2 % sulfur removal of kerosene is achieved after 3.5 min oxidation followed by liquid–liquid extraction in the absence of ultrasound irradiation. The observed trend can be attributed to the physical effect of the ultrasound to produce intense micromixing and emulsification of the present biphasic oxidation system (Bolla et al. 2012).
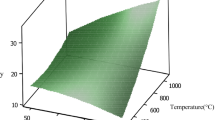
Figure 5 shows the effect of oxidation temperature on the sulfur removal under the optimal conditions.
Effect of oxidation temperature on the sulfur removal of kerosene. Oxidation conditions: n o/n s = 15.02, n acid/n s = 107.8, ultrasound power per kerosene volume = 7.6 W/mL, and sonication time = 10.5 min. Extraction conditions: ambient temperature; solvent: acetonitrile with solvent-to-kerosene volume ratio = 1; no. of extraction stages = 1
As can be observed, an increase in the oxidation temperature up to 50 °C leads to an increase in the sulfur removal, although further increase in the temperature beyond this limit leads to a slight decrease in the sulfur removal. It should be noted that increasing the oxidation temperature has two contrary effects. On one hand it leads to an increase in the rate of oxidation of different sulfur-containing compounds of kerosene (Sobati et al. 2010c), which has a positive effect on the sulfur removal. On the other hand, it decreases the equilibrium concentration of peroxyformic acid due to thermal decomposition (Xiaoying et al. 2011), which may have a negative effect on the sulfur removal. The higher oxidation temperature also leads to the greater vapor pressure of the volatile compounds in the oxidation media that in turn facilitates bubble formation and reduces the cavitation threshold and micromixing intensity, which have negative effects on the sulfur removal. It seems that the former effect is dominant up to 50 °C and the latter effects become pronounced in the temperature range beyond 50 °C.
Effect of the number of extraction stages and reduction of hydrocarbon loss
It should be noted that there are several candidates for the extraction solvent. According to Sobati et al., acetonitrile is an appropriate solvent for the extraction of the oxidized sulfur-containing compounds of kerosene (Sobati et al. 2010b). The main advantage of acetonitrile is its low boiling point (i.e., 82 °C) in comparison with boiling points of the oxidized sulfur-containing compounds (i.e., 277–677 °C), which facilitates the solvent recovery by applying distillation (Sobati et al. 2010a).
Larger values of the sulfur removal is expected if more extraction stages are applied because of the improvement in the efficiency of extraction of oxidized sulfur-containing compounds. However, this causes less kerosene recovery due to transfer of greater amount of dissolved kerosene into the extraction solvents.
Table 8 shows the sulfur removal and recovery of kerosene after extraction as a function of the number of extraction stages. In addition, the values of sulfur removal using a single extraction and without oxidation as well as the sulfur removal after ultrasound-assisted oxidation and without any extraction stage are presented in this table.
Table 8 indicates as the number of applied extraction stages is increased from 1 to 5 (after oxidation) the sulfur removal is also raised from 95.46 to 98 % and the kerosene recovery is reduced from 90 to 55 %.
In other words, increasing the number of extraction stages beyond 1 has little effect on the final sulfur removal, while it leads to a continuous decrease in the kerosene recovery. Extraction effectiveness factor (E) is introduced in order to combine the sulfur removal and kerosene recovery in a single parameter:
Figure 6 shows the extraction effectiveness factor as a function of the number of extraction stages.
Extraction effectiveness factor, E, versus the number of extraction stages. Oxidation conditions: n o/n s = 15.02, n acid/n s = 107.8, ultrasound power per fuel oil volume = 7.6 W/mL, sonication time = 10.5 min, and T = 50 °C. Extraction conditions: ambient temperature; solvent: acetonitrile with solvent-to-kerosene volume ratio = 1
Figure 6 shows the extraction effectiveness factor is reduced continuously as the number of extraction stages is increased. It was tried to improve the kerosene recovery after extraction by solvent washing. In this context, the extract obtained after extraction was washed with double volume distilled water, and the hydrocarbon obtained after phase separation was mixed with the treated kerosene. As can be observed in Table 8, kerosene recovery is increased from 90 to 98 % and the sulfur removal is decreased from 95.46 to 87.95 % in comparison with the conditions where oxidation is followed by a single stage extraction. Water addition step leads to a decrease in the solubility of hydrocarbons in water/acetonitrile mixture and the production of a secondary hydrocarbon layer after phase separation, which in turn increases the kerosene recovery.
In order to investigate the role of the oxidation in the final sulfur removal, the sulfur removal of kerosene by a single extraction process is compared with the sulfur removal obtained when the oxidation is followed by the solvent extraction in the same extraction condition. As can be observed in Table 8, the single extraction process leads to only 29.9 % sulfur removal, while applying the oxidation before the extraction process increases the sulfur removal up to 95.46 %. It should be noted that in a single extraction, the polarity of the original sulfur-containing compounds of kerosene is not much higher than that of the corresponding hydrocarbon compounds, and the extraction is not efficient.
Furthermore, Table 8 indicates 74.9 % of the total sulfur of kerosene is removed after ultrasound-assisted oxidation. This has been achieved by simultaneous extraction of the oxidized sulfur-containing compounds to the aqueous phase (i.e., formic acid and hydrogen peroxide mixture) in the oxidation media. It can be concluded that in the UAOD process, a major sulfur removal of the kerosene is carried out through in situ extraction of the oxidized sulfur-containing compounds into the aqueous phase of the oxidation media. The polarities of the remaining oxidized sulfur-containing compounds are changed in such a way that they can be extracted more effectively. In other words, there is a synergistic effect between the oxidation and extraction in the UAOD process.
It was also tried to recover the spent acetonitrile by applying the distillation process. The recovered acetonitrile was used for the extraction of the oxidized sulfur-containing compounds of kerosene. The performance of recovered acetonitrile was very good, and only 0.1 % reduction in the sulfur removal was observed using the first recovered acetonitrile in comparison with the fresh acetonitrile. It should be noted that only 0.5 % reduction in the sulfur removal was observed after three distillation recovery cycles of acetonitrile. In addition, 7 % of solvent volume was decreased after each distillation recovery cycle. In other words, about 7 % fresh solvent make up is needed after distillation.
Effect of the UAOD on the kerosene specifications
Table 2 shows the major properties of the kerosene before and after UAOD treatment. The distillation characteristics of the kerosene before and after UAOD process were obtained according to ASTM-D86 in order to investigate whether the properties of the treated kerosene are negatively affected by the UAOD process. As can be observed, the distillation characteristics of the treated and untreated kerosene remain approximately unchanged. As can be observed in Table 2, the aromatic content of kerosene decreases from 15.9 to 10.9 vol%. Due to the dearomatization of kerosene, the naphthene and olefin contents of the treated kerosene are slightly increased. The total water content of the untreated and treated kerosene was also measured by Karl-Fischer titration. Table 2 also indicates a negligible increase in the water content of the kerosene after UAOD treatment.
Figure 7 displays the comparison of the GC-FPD chromatograms of the treated kerosene with untreated kerosene.
GC-FPD chromatograms of the untreated and treated kerosene: a untreated kerosene; b oxidized kerosene; c treated kerosene after oxidation followed by extraction. Oxidation conditions: n o/n s = 15.02, n acid/n s = 107.8, ultrasound power per fuel oil volume = 7.6 W/mL, sonication time = 10.5 min, and T = 50 °C. Extraction conditions: ambient temperature; solvent: acetonitrile with solvent-to-kerosene volume ratio = 1
As can be observed, the peaks of the sulfur-containing compounds were diminished in the treated kerosene.
Conclusions
An experimental investigation was conducted on the UAOD of non-hydrotreated kerosene using oxidation with hydrogen peroxide-formic acid followed by liquid–liquid extraction using acetonitrile. The response surface methodology (RSM—Box–Behnken design) was employed for the experimental design. The best operating conditions were obtained using RSM method. The maximum sulfur removal was achieved using (n o/n s) of 15.02, (n acid/n s) of 107.8, and the ultrasound power per fuel oil volume of 7.6 W/mL. Sulfur removal of 95.4 % was achieved under these oxidation conditions followed by the liquid–liquid extraction.
A valid, quadratic predictive correlation between the operating variables of the oxidation and the sulfur removal was developed using RSM. The obtained mathematical correlation from the data analysis gave a good prediction at high significant level.
The required reaction time of the UAOD was significantly decreased in comparison with the conventional ODS due to the cavitation phenomenon and subsequent enhancement in the interphase mass transfer in the biphasic oxidation system in the absence of the expensive phase-transfer agents. As an important improvement, more than 90 % sulfur removal was gained in shorter than 3.5-min sonication time followed by a liquid–liquid extraction step, whereas the observed sulfur removal using conventional ODS in the identical conditions was 70.2 %. According to Gonzalez et al., 98 % sulfur removal of jet fuel was achieved after a long oxidation time (i.e., 120 min) using ultrasound-assisted formic acid/hydrogen peroxide/activated carbon oxidation system (Gonzalez et al. 2012). In other words, the required oxidation time was decreased significantly in the present UAOD system in comparison with other similar studies. It was also found that 74.9 % of the total sulfur of the kerosene was removed after ultrasound-assisted oxidation under the optimal condition.
The present UAOD process can be applied as an alternative or complementary process for the HDS process due to its great advantages such as simplicity, mild operating conditions, no hydrogen consumption, and no need to use a special catalyst.
Abbreviations
- D :
-
Percentage of desulfurization, % in Eq. (6)
- E :
-
Solvent effectiveness factor that depends on the desulfurization in Eq. (6)
- R :
-
Kerosene recovery after extraction, % in Eq. (6)
- H+ :
-
Hydrogen ion
- n o :
-
Moles of oxidant (i.e., hydrogen peroxide)
- n acid :
-
Moles of acid (i.e., formic acid)
- n s :
-
Moles of total sulfur
- β :
-
Constant offset in Eq. (1)
- X :
-
Coded independent variables in Eq. (1)
- Ε:
-
Unanticipated error in Eq. (1)
- Y :
-
Predicted response
References
Abdullah WNW, Bakar WAWA, Ali R, Embong Z (2014) Oxidative desulfurization of commercial diesel catalyzed by tert-butyl hydroperoxide polymolybdate on alumina: optimization by Box–Behnken design. Clean Technol Environ Policy 17:433–441
Akbari A, Omidkhah M, Darian JT (2014) Investigation of process variables and intensification effects of ultrasound applied in oxidative desulfurization of model diesel over MoO3/Al2O3 catalyst. Ultrason Sonochem 21:692–705
Al-Lal A-M, Bolonio D, Llamas A, Lapuerta M, Canoira L (2015) Desulfurization of pyrolysis fuels obtained from waste: lube oils, tires and plastics. Fuel 150:208–216
Bhasarkar JB, Chakma S, Moholkar VS (2013) Mechanistic Features of oxidative desulfurization using sono-Fenton–peracetic acid (ultrasound/Fe2+–CH3COOH–H2O2) system. Ind Eng Chem Res 52:9038–9047
Bhasarkar JB, Chakma S, Moholkar VS (2015a) Investigations in physical mechanism of the oxidative desulfurization process assisted simultaneously by phase transfer agent and ultrasound. Ultrason Sonochem 24:98–106
Bhasarkar JB, Singh M, Moholkar VS (2015b) Mechanistic insight into phase transfer agent assisted ultrasonic desulfurization. RSC Adv 5:102953–102964
Bolla MK, Choudhury HA, Moholkar VS (2012) Mechanistic features of ultrasound-assisted oxidative desulfurization of liquid fuels. Ind Eng Chem Res 51:9705–9712
Calcio Gaudino E et al (2014) Efficient H2O2/CH3COOH oxidative desulfurization/denitrification of liquid fuels in sonochemical flow-reactors. Ultrason Sonochem 21:283–288. doi:10.1016/j.ultsonch.2013.04.009
Campos-Martin J, Capel-Sanchez M, Fierro J (2004) Highly efficient deep desulfurization of fuels by chemical oxidation. Green Chem 6:557–562
Campos-Martin JM, Capel-Sanchez M, Perez-Presas P, Fierro J (2010) Oxidative processes of desulfurization of liquid fuels. J Chem Technol Biotechnol 85:879–890
Choi AES, Roces S, Dugos N, Futalan CM, Lin S-S, Wan M-W (2014) Optimization of ultrasound-assisted oxidative desulfurization of model sulfur compounds using commercial ferrate (VI). J Taiwan Inst Chem Eng 45:2935–2942. doi:10.1016/j.jtice.2014.08.003
Choi AES, Roces S, Dugos N, Wan M-W (2015) Operating cost study through a Pareto-optimal fuzzy analysis using commercial ferrate (VI) in an ultrasound-assisted oxidative desulfurization of model sulfur compounds. Clean Technol Environ Policy. doi:10.1007/s10098-015-1079-6
Dagaut P, El Bakali A, Ristori A (2006) The combustion of kerosene: experimental results and kinetic modelling using 1-to 3-component surrogate model fuels. Fuel 85:944–956
De Filippis P, Scarsella M, Verdone N (2009) Peroxyformic acid formation: a kinetic study. Ind Eng Chem Res 48:1372–1375
De Filippis P, Scarsella M, Verdone N (2010) Oxidative desulfurization I: peroxyformic acid oxidation of benzothiophene and dibenzothiophene. Ind Eng Chem Res 49:4594–4600
De Filippis P, Liuzzo G, Scarsella M, Verdone N (2011) Oxidative desulfurization II: temperature dependence of organosulfur compounds oxidation. Ind Eng Chem Res 50:10452–10457
Dehkordi AM, Kiaei Z, Sobati MA (2009a) Oxidative desulfurization of simulated light fuel oil and untreated kerosene. Fuel Process Technol 90:435–445
Dehkordi AM, Sobati MA, Nazem MA (2009b) oxidative desulfurization of non-hydrotreated kerosene using hydrogen peroxide and acetic acid. Chin J Chem Eng 17:869–874. doi:10.1016/S1004-9541(08)60289-X
Dehkordi AM, Sobati M, Nazem MA (2013) An experimental investigation on the oxidative desulfurization of kerosene feedstock. Energy Sources Part A 35:226–234
Etemadi O, Yen TF (2007) Selective adsorption in ultrasound-assisted oxidative desulfurization process for fuel cell reformer applications. Energy Fuels 21:2250–2257
Gonzalez LA, Kracke P, Green WH, Tester JW, Shafer LM, Timko MT (2012) Oxidative desulfurization of middle-distillate fuels using activated carbon and power ultrasound. Energy Fuels 26:5164–5176
Hajamini Z, Sobati MA, Shahhosseini S, Ghobadian B (2016) Waste fish oil (WFO) esterification catalyzed by sulfonated activated carbon under ultrasound irradiation. Appl Therm Eng 94:141–150
Ismagilov Z et al (2011) Oxidative desulfurization of hydrocarbon fuels. Catal Rev 53:199–255
Kadijani JA, Narimani E, Kadijani HA (2015) Using response surface methodology to optimize ultrasound-assisted oxidative desulfurization. Korean J Chem Eng. doi:10.1007/s11814-015-0276-7
Kuppa R, Moholkar VS (2010) Physical features of ultrasound-enhanced heterogeneous permanganate oxidation. Ultrason Sonochem 17:123–131
Liu L, Zhang Y, Tan W (2014) Ultrasound-assisted oxidation of dibenzothiophene with phosphotungstic acid supported on activated carbon. Ultrason Sonochem 21:970–974
Lu M-C, Biel LCC, Wan M-W, de Leon R, Arco S (2014) The oxidative desulfurization of fuels with a transition metal catalyst: a comparative assessment of different mixing techniques international. Int J Green Energy 11:833–848. doi:10.1080/15435075.2013.830260
Margeta D, Sertić-Bionda K, Foglar L (2016) Ultrasound assisted oxidative desulfurization of model diesel fuel. Appl Acoust 103:202–206
Montgomery DC, Runger GC (2010) Applied statistics and probability for engineers. Wiley, New York, pp 277–326
Nunes MA et al (2014) Evaluation of nitrogen effect on ultrasound-assisted oxidative desulfurization process. Fuel Process Technol 126:521–527
Shakirullah M, Ahmad W, Ahmad I, Ishaq M (2010) Oxidative desulphurization study of gasoline and kerosene: role of some organic and inorganic oxidants. Fuel Process Technol 91:1736–1741
Sobati MA, Dehkordi AM, Shahrokhi M (2010a) Extraction of oxidized sulfur-containing compounds of non-hydrotreated gas oil. Chem Eng Technol 33:1515–1524. doi:10.1002/ceat.200900622
Sobati MA, Dehkordi AM, Shahrokhi M (2010b) Liquid–liquid extraction of oxidized sulfur-containing compounds of non-hydrotreated kerosene. Fuel Process Technol 91:1386–1394
Sobati MA, Dehkordi AM, Shahrokhi M, Ebrahimi AA (2010c) Novel type of four-impinging-jets reactor for oxidative desulfurization of light fuel oils. Ind Eng Chem Res 49:9339–9348
Srivastava VC (2012) An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv 2:759–783
Villasenor F, Loera O, Campero A, Viniegra-Gonzalez G (2004) Oxidation of dibenzothiophene by laccase or hydrogen peroxide and deep desulfurization of diesel fuel by the later. Fuel Process Technol 86:49–59
Wan M-W, Yen T-F (2007) Enhance efficiency of tetraoctylammonium fluoride applied to ultrasound-assisted oxidative desulfurization (UAOD) process. Appl Catal A 319:237–245
Wang D, Qian EW, Amano H, Okata K, Ishihara A, Kabe T (2003) Oxidative desulfurization of fuel oil: part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide. Appl Catal A 253:91–99
Xiaoying S, Xuebing Z, Wei D, Dehua L (2011) Kinetics of formic acid-autocatalyzed preparation of performic acid in aqueous phase. Chin J Chem Eng 19:964–971
Yang RT, Hernández-Maldonado AJ, Yang FH (2003) Desulfurization of transportation fuels with zeolites under ambient conditions. J Sci 301:79–81
Žnidarčič A, Mettin R, Dular M (2015) Modeling cavitation in a rapidly changing pressure field—Application to a small ultrasonic horn. Ultrason Sonochem 22:482–492
Acknowledgments
The authors would like to acknowledge the supports provided by the National Iranian Oil Engineering and Construction Company (NIOEC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khodaei, B., Sobati, M.A. & Shahhosseini, S. Optimization of ultrasound-assisted oxidative desulfurization of high sulfur kerosene using response surface methodology (RSM). Clean Techn Environ Policy 18, 2677–2689 (2016). https://doi.org/10.1007/s10098-016-1186-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-016-1186-z