Abstract
Sawdust, SD, was reacted with Sandene 850 (polyamine) in alkaline medium to yield cationized sawdust. The latter was characterized through its Fourier Transform Infrared (FT-IR) spectra and nitrogen content. Thus, obtained cationized sawdust was harnessed for removal of Direct Red 23 from aqueous solutions. Adsorption studies were performed under different agitation time, adsorbent and adsorbate concentrations and the onset of this on the adsorption capacity of Direct Red 23 onto cationized sawdust. Langmuir and Freundlich models were applied in the adsorption studies. The study showed that the cationized sawdust is effectively used in adsorption of Direct Red 23 from aqueous solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effluents from the dyestuff manufacturing and textile industries, in particular, are highly colored with a large amount of suspended organic solids and considered as important sources of water pollution. When the quality of the environment is considered, removal of synthetic dyes becomes a must, because some of these dyes and their degradation products may be carcinogenic and toxic. Consequently, their treatment cannot solely depend on biodegradation alone (Pagga and Braun 1986). Indeed, removal of dyes is nowadays regarded as an important practice in textile wastewater treatment.
Numerous physicochemical and biological methods have been used to decolorize dye bearing effluents, often in combinations as one single treatment may not be sufficient to remove certain classes of synthetic dyes (Leitch and Armstrong 2006; Forgacs et al. 2004). Adsorption has some advantages over other methods and is considered a versatile technology because very high levels of color removal can often be attained by using an appropriate adsorbent. Various types of activated carbon (Pereira et al. 2003; Faria et al. 2004) and polymeric ion-exchange resin (Karcher et al. 2001; Karcher et al. 2002) have been used to remove a wide range of dyes from water. However, these adsorbents are generally expensive. As a consequence, there has been considerable effort directed toward development of low-cost adsorbents for color removal (Sanghi and Bhattacharya 2002). The efficacy of these adsorbents, however, varies from one material to another.
Previous work in this division has been devoted to utilize modified cellulose (Hashem and El-shishtawy 2001; Aly et al. 2005) and plant residues such as sawdust (Hashem et al. 2006), cotton stalks (Hashem et al. 2006), as well as cellulosic fabric wastes (Sokker et al. 2005) for the removal of dyes from aqueous solutions. Special emphasis was placed on utilization of cationized sawdust for the removal of Zn(II) from aqueous solution (Hashem et al. 2007). The present work is undertaken with a view to study the technical feasibility of the cationized sawdust for removal of direct dyes, exemplified by Direct Red 23, from aqueous solution.
To achieve the goal, sawdust was reacted with sandene and the cationized sawdust obtained was characterized using FTIR spectroscopy. The so synthesized modified sawdust was used as a low-cost absorbent for the said direct dye from aqueous solutions at different conditions and; the extent to which the experimental data obeyed the Langmuir and Freundlich isotherms was clarified.
Experimental
Materials
Wood sawdust with a particle size range of 125–200μm was used. The chemical characteristics of sawdust are shown in Table 1. Sandene 850, (Polyamine) having a nitrogen percent of 5.82 % was supplied by Sandoz Company, Switzerland.
Dyestuffs
Direct Red 23 dye was supplied by ISMA dye Company, Egypt and was used without further purification. The structure of this dye is shown in Scheme 1.
Cationization of sawdust
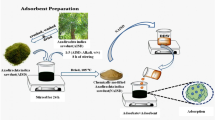
Sawdust (4 g) with mesh size of 125–200 μm was mixed well with the required amount of NaOH (0.246 mol/g of sawdust) in a 100 ml Erlenmeyer flask. The required amount of Sandene was added (1 ml/g of sawdust) to the flask under continuous mixing with a spatula until a homogeneous paste was obtained. The resulting reaction mixture was maintained in a thermostatic water-bath at a specified temperature and reaction time. After completion of the reaction, the resulting mixture was neutralized by treatment with acidified ethanol, the precipitated product being washed several times with distilled water to remove the non reacted Sandene, followed by washing with acetone and then dried in an electric oven at 333 K for 3 h.
Adsorption experiments
Aqueous solutions of Direct Red 23 were prepared by dissolving the dye in distilled water to the required concentrations. Certain amount of cationized sawdust powder and 100 ml of an aqueous dye solution were placed in a 125 ml glass Stoppard flask and stirred for 3 h using a shaking water-bath operated at 200 rpm at 303 K. The samples were withdrawn from the shaker, and the dye solution was separated from the adsorbent by centrifugation. Dye concentration in the supernatant solution was measured with a Shimadzu UV–Visible spectrophotometer. The amount of dye adsorbed onto cationized sawdust, q e (mg/g), was calculated by the mass balance relationship (Eq. 1) as follows:
where C 0 and C e are the initial and equilibrium liquid phase concentrations of dye, respectively (mg /l). V is the volume of solution (l), and W is the weight of the adsorbent (gm).
Analysis
Fourier transform infrared (FT-IR) spectroscopy
The FT-IR spectra of sawdust and cationized sawdust were recorded on a Perkin-Elmer Spectrum 1000 spectrophotometer over the range of 4000–400 cm−1 using KBr disc technique.
Nitrogen content
The percent nitrogen of cationized sawdust was determined according to the micro Kjeldahl method.
Results and discussion
Sandene reacts with the cellulose of sawdust in alkaline medium according to the scheme suggested below:

Cationized sawdust is in the form of a brown powder having a N% of 5.4% against nitrogen % of 0.1 for unmodified sawdust.
FT-IR spectra
Figure 1a, b shows the FT-IR spectra of native sawdust and cationized sawdust, respectively. It can be seen that the characteristic absorption peak of OH group of sawdust cellulose is located at 3406.76 cm−1. This peak becomes wider and broader and shifts from 3423.54 to 3343.02 cm−1 in the case of modified sawdust. This can be attributed to the presence of absorption of NH stretching at 3400 cm−1 that overlaps with the absorption peak of OH group (in the range of 3200–3500 cm−1).
Factors affecting adsorption of Direct Red 23 on cationized sawdust
Effect of agitation time
Figure 2 shows the effect of contact time at 303 K on the adsorption capacity, q e (mg/g), on cationized sawdust using the latter at a concentration of 2 g/l at and Direct Red 23 at a concentration of 150 mg/l at pH 5.5. It is clear that the increase of agitation time from 0 to 90 min is accompanied by an increase in the adsorption capacity, q e, of Direct Red 23 onto cationized sawdust. A further increase in the agitation time has practically no effect on the q e. That is, the equilibrium is attained after 90 min, within the concentration range studied. It is further observed that the adsorption curves are continuous, which indicates the possibility of the formation of monolayer coverage of Direct Red 23 onto the adsorbent surface. It is logical that the favorable effect of agitation time is a manifestation of proving better opportunity for interaction between the adsorbent, i.e., cationized sawdust and the adsorbate, i.e., Direct Red 23.
Effect of adsorbent concentration
The effect of the adsorbent concentration on both percent removal and adsorption capacity, q e, of Direct Red 23 onto cationized sawdust was studied by varying the concentration of the adsorbent from 1 to 5 g/l with a constant initial dye concentration of 150 mg/l, agitation time of 120 min at 303 K and at fixed pH. The results are shown in Fig. 3. It is clear that the adsorption capacity decreases from 79.8 mg/g (53.2%) at 1 g/l dose to 34.9 mg/g (93 %) at 5 g/l dose for Direct Red 23 cationized sawdust, indicating that a dose of 1 g/l of adsorbent is sufficient for the optimum for adsorption of Direct Red 23 from aqueous solutions. The increase in percent removal of Direct Red 23 from 53.2% to 93% by increasing the adsorbent concentration from 1 to 5 g/l could be attributed to the availability of increasing number of exchangeable sites. This is a common observation whenever either the number of adsorption sites or the active surface area is increased (Hashem et al. 2007; Yu et al. 2000). However, the amount of dye adsorbed per unit mass of the adsorbent (q e) at equilibrium decreases with an increase in the adsorbent concentration. The decrease in adsorption capacity from 79.8 to 34.9 mg/g by increasing the adsorbent dose from 1 to 5 g/l is mainly due to overlapping of the adsorption sites as a result of overcrowding of the adsorbent particles and is also due to the competition among dye molecules on the adsorbent surface (Kadirvelu and Namasivayam 2003).
Adsorption isotherm
The adsorption isotherm indicates how the adsorbate molecules are distributed between the liquid phase and solid phase when the adsorption process reaches an equilibrium state. The analysis of the isotherm data by fitting them to different isotherm models is an important step to find the suitable model that can be used for design purposes (El-Guendi 1991). Figure 4 shows the adsorption isotherm of Direct Red 23 onto cationized sawdust at 303 K and at pH 6.5. Adsorption data are the most conveniently described by adsorption isotherms, such as Langmuir or Freundlich isotherms which relate adsorption capacity, qe (dye uptake per unit weight of adsorbent), to equilibrium adsorbate concentration in the bulk fluid phase, C e.
The data of Fig. 4 show the affinity of Direct Red 23 on cationized sawdust. They highlight the behavior which may be attributed to coulombic attraction between the cationic group (resulted from amino group in acid medium) in modified sawdust and the negative dye species of Direct Red 23. The isothermal equation data were processed employing Langmuir and Freundlich equations.
The Langmuir equation (Langmuir 1916) is given by:
where C e is the equilibrium concentration of adsorbate (mg/l), q e is the amount of the Direct Red 23 adsorbed (mg/g) on cationized sawdust. Q max (mg/g) and b (l/mg) are the Langmuir constants related to the adsorption capacity and energy of adsorption, respectively. Q max represents a maximum adsorption capacity.
When C e/q e was plotted against C e, a straight line with the slope of 1/Q max was obtained. Q max and b were determined from the slope and intercept of the plot and are presented in Table 2. Figure 5 shows the Langmuir adsorption plots of Direct Red 23 on cationized sawdust at 303 K.
The fit in this case (R 2 = 0.9948) indicates the applicability of the Langmuir model (Eq. 2) for the system under investigation; the results also demonstrate monolayer coverage of Direct Red 23 at the outer surface of the cationized sawdust. It will be seen from Fig. 5 that the maximum adsorption capacity, Q max, of Direct Red 23 on cationized sawdust is 65.8 mg/g. One of the essential characteristics of the Langmuir isotherm could be expressed in terms of a dimensionless constant separation factor or equilibrium parameter, R L, which describes the type and shape of the isotherm (Hall et al. 1966) as summarized in Table 3 and defined by Eq. 3:
where b (l/mg) is the Langmuir constant related to the energy of adsorption and C 0 (mg/l) is the initial concentration of Direct Red 23. As is evident from Table 4, all R L values were between 0.0303 and 0.2381 for Direcr Red 23 by cationized sawdust. All these values were between 0 and 1 and indicate the favorable adsorption of Direct Red 23 on cationized sawdust.
The Freundlich isotherm assumes that the uptake of dyes occurs on a heterogeneous surface by multilayer adsorption. The Freundlich equation (Freundlish 1906), which was also applied for the adsorption of dye, is given by:
where q e is the amount of adsorbate adsorbed per unit weight (mg/g) of adsorbent, C e is the equilibrium concentration of Direct Red 23 (mg/l), and K F (mg/g) and n are Freundlich constants related to the capacity of adsorption and favorability of adsorption, respectively.
Linear plots of log q e versus log C e give a straight line, the slope and intercept of which correspond to 1/n and log K F, respectively. Freundlich plot for Direct Red 23 on cationized sawdust are shown in Fig. 6. The values of Freundlich constants are listed in Table 5. The value of n is 2.56 for Direct Red 23 on catiobnized sawdust (0 < n < 10), showing that the adsorption of Direct Red 23 on cationized sawsust is favorable. The Langmuir equation is better obeyed by the system than the Freundlich one, as is evident by the values of correlation coefficients shown in Tables 2 and 5.
Conclusion
Cationized sawdust was prepared by the reaction of sawdust with Sandene in alkaline medium. The cationized sawdust was characterized by FT-IR spectroscopy and estimation of nitrogen content. The so obtained modified sawdust was used for adsorption of Direct Red 23 from aqueous solution. The experimental data showed that the cationized sawdust was is very adequate for removal of Direct Red 23 from aqueous solution. The adsorption data of Direct Red 23 onto cationized sawdust obey the Langmuir and Freundlich isotherms.
References
Aly AS, Sokker HH, Hashem A, Hebeish A (2005) Preparation of cellulosic membrane containing pyrrolidone moiety via radiation induced grafting and its application in wastewater treatment. Am J Appl Sci 2:508–513
El-Guendi M (1991) Adsorption of basic dye using activated carbon prepared from oil palm. Adsorpt Sci Technol 8:217–225
Faria PCC, Orfao JJM, Pereira MFR (2004) Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res 38:2043–2052
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Freundlish H (1906) Over the adsorption in solution. J Phys Chem 57:385–470
Hall KR, Eagleton LC, Acrivos A, Vermevlem T (1966) Pore and solid diffusion kinetics in fixed bed adsorption under constant pattern conditions. Ind Eng Chem Fundam 5:212–223
Hashem A, El-shishtawy RM (2001) Preparation and characterization of cationized cellulose for the removal of anionic dyes. Adsorpt Sci Technol 19:197–202
Hashem A, Aly AA, Aly AS (2006a) Preparation and utilization of cationized sawdust. Polym Plastics Technol Eng 45:395–401
Hashem A, Aly AA, Aly AS, Hebeish A (2006b) Quaternization of cotton stalks and palm tree particles for removal of acid dye from aqueous solutions. Polym Plast Technol Eng 45:389–394
Hashem A, Abdel-Halim E, Maauof HA, Ramadan MA, Abo-Okeil A (2007) Treatment of sawdust with polyamine for wastewater treatment. Energy Educ Sci Technol 19:45–58
Kadirvelu K, Namasivayam C (2003) Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd(II) from aqueous solution. Adv Environ Res 7:471–478
Karcher S, Kornmüller A, Jekel M (2001) Screening of commercial sorbents for the removal of reactive dyes. Dyes Pigments 51:111–125
Karcher S, Kornmüller A, Jekel M (2002) Anion exchange resins for removal of reactive dyes from textile wastewaters. Water Res 36:4717–4724
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Leitch AE, Armstrong PB, Chu KH (2006) Characteristics of dye adsorption by pretreated pine bark adsorbents. Int J Environ Stud 63:59–66
Pagga U, Braun D (1986) The degradation of dye stuffs. Part II. Behaviour of dyestuffs in aerobic biodegradation tests. Chemosphere 15:479
Pereira MFR, Soares SF, Orfao JJM, Figueiredo JL (2003) Adsorption of dyes on activated carbons: influence of surface chemical groups. Carbon 41:811–821
Sanghi R, Bhattacharya B (2002) Review on decolorization of aqueous dye solutions by low cost adsorbents. Color Technol 118:256–269
Sokker HH, Abdel-Halim ES, Aly AS, Hashem A (2005) Cellulosic fabric wastes grafted with DMAEMA for the removal of direct dyes. Adsorpt Sci Technol 22:679–691
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater B80:33–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hebeish, A., Ramadan, M.A., Abdel-Halim, E. et al. An effective adsorbent based on sawdust for removal of direct dye from aqueous solutions. Clean Techn Environ Policy 13, 713–718 (2011). https://doi.org/10.1007/s10098-010-0343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-010-0343-z