Abstract
In this case study, the process modifications and improvement to the existing process at the Emirates Gold refinery (in order to meet the United Arab Emirates (UAE) nitrogen oxides air emission regulations) is presented. In the past, Emirates Gold refinery used a single small scrubber to treat waste gases. In order to treat the waste gas efficiently, it was found that a cooled oxidation reactor (oxidizer) before the existing scrubber, as well as a second scrubber is needed. The waste gas is mixed with air at a fixed ratio before entering the oxidizer which is designed to obtain the optimum degree of NO oxidation (about 50%).To keep the oxidation reactions in the desirable direction the temperature should be kept between 15 and 20°C There for an internal cooler was required. The gas mixture from the Oxidizer enters the first scrubber (existing) where most of the NO x , mainly as N2O3, are absorbed by a NaOH solution (15–20%). The remaining NO x , mainly as N2O3 is absorbed in the second scrubber by a NaOH solution (8–10%). The mass transfer area of the packing in the two scrubbers, the solution circulation rate, and the cooling duty were designed to reach the desired degree of absorption of N2O3 and NO2. This ensures that the recommended NO x residual value of 500 mg/m3 (250 ppm) is reached. All reactions occur simultaneously was calculated using EQ4WIN software. The data obtained for different temperatures was processed with Stat View, SuperPro Designer simulation and Aspen HYSYS simulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emirates Gold is the largest gold and silver refinery in the Arabian Gulf, equipped with hi-tech refinery facilities with a capacity of 2,500 kg/day. The most important step in the gold refining process is dissolving the scrap gold in aqua regia (Latin for royal water); it is a highly corrosive solution that is fuming yellow or red in color. Due to this corrosive nature, QVF glassware was used as the material of construction in all units. The solution is formed by mixing concentrated nitric acid and concentrated hydrochloric acid, usually in a volumetric ratio of 1:3 (Massucci et al. 1999). Nitric acid is a strong oxidizer, which will dissolve gold completely, forming Au3+. The hydrochloric acid provides the needed Cl−, which reacts with gold to produce chloraurate anions as shown in reaction (1).
The dissolution of gold takes place in a semi-batch reactor with a normal-operating capacity of 125-kg scrap gold. The cycle time of each batch is approximately 90 min. Nitrogen oxide (NO) is formed by the basic reaction of the scrap gold dissolution. In addition, nitrogen dioxide (NO2) is produced, if oxygen is present in the gas phase. Nitrogen oxide compounds (NO x , i.e., NO and NO2) are known to be major atmospheric pollutants. NO is one of the most harmful air pollutants. NO x contributes to acid rain and greenhouse effects, which are of great concern. Concentrations of NO x in the air have also been linked to mortality rates, where it was found that an increase in concentration by 10 μg/m3 lead to, on average, a 0.4% increase in mortality (Qian et al. 2010).
Regulations concerning limitation of air emission of NO x from industrial processes differ from one country to another. The UAE regulatory limits NO x emissions, including averaging times, to a maximum of 500 mg/m3 NO x (without abatement systems) (Dubai Municipality 2008). Due to the change in the UAE regulation of air pollutant emission, it was necessary that the Emirates Gold refinery improve its waste gas treatment process to meet the stringent requirement. There are several methods for controlling NO x emissions such as selective catalytic reduction (SCR) or selective non-catalytic reduction (SNCR) (Lietti 1996; Heidenreich et al. 2008). The most common forms of NO x treatment is via gas scrubbing using an alkaline solution (Chironna and Altshuler 2009; Thomas and Vanderschuren 2000; Patwardhan and Joshi 2004; Coates 1987). Conventional gas scrubbing usually utilizes five alkaline scrubbers in series (Günther 2010). Other scrubbing solutions are available, such as hydrogen peroxide and ammonia (Wallin et al. 2003; Economidis et al. 1998). In the case of the hydrogen peroxide, it acts as an oxidizer as well as the absorber of the NO x (Thomas and Vanderschuren 1996). Several new studies employs bio-treatment for NO x contaminated gas by the application of denitrification (Flanagan et al. 2002; Barnes et al. 1995; Du Plessis et al. 1998).
Objective
The existing treatment process for the waste gas at Emirates Gold employs a single scrubbing column using sodium hydroxide (NaOH) as scrubbing solution (Fig. 1). The chemistry controlling the treatment process is as follows:
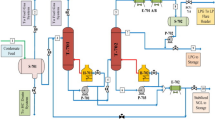
Flow sheet of the waste gas treatment system. 1 Waste gas from gold scrap dissolution process, 2 Air to be mixed with stream (1), 3 Mixed gas feed to oxidizer (see Table 9), 4 Effluent gas from oxidizer (see Table 9), 5 NaOH (15–20%) feed to the first scrubber, 6 Treated gas effluent from the first scrubber, 7 Liquid outlet from the first scrubber, 8 Recycle stream of NaOH, 9 Recycled NaOH from the first scrubber to the second scrubber, 10 Recycled NaOH from the second scrubber, 11 Recycle stream to the second scrubber (8–10% NaOH), 12 Purified gas from the second scrubber (see Table 9), 13 Liquid outlet from the second scrubber, 14 Purge stream from second scrubber
Nonetheless, the process is inefficient and does not meet the UAE regulations for NO x air emissions. Since the ratio of NO to NO2 in the exhaust stream is significant and a high NO removal efficiency is required, multiple scrubbing stages are essential. In this paper, improvements and modifications to the current treatment system that is utilized at the Emirates Gold refinery in order to meet the UAE air emission regulations are presented.
The modified process
As stated above, sodium hydroxide is used as the scrubbing solution for NO x treatment. The absorption rate constants are higher if diluted NaOH solutions are used. To reach a high degree of absorption as well as very low residual concentrations of NO x in the treated gas mixture, dual alkali scrubbers (absorption columns) packed with raschig rings in series, different concentrations of the alkali solutions were proposed. A schematic diagram of the modified process to treat NO x is shown in Fig. 1. The proposed modification includes an oxidizer, first scrubber, and second scrubber. The oxidizer is the first operation and the second scrubber is the last step. NO has a very low solubility in NaOH, hence posing a significant problem for its absorption. However, if the NO is oxidized to other NO x it becomes much more soluble (Thomas and Vanderschuren 1996). Several chemical additives have been suggested to overcome this issue of insolubility, such as ClO2 or Ozone (Chien and Chu 2000). However, such chemical additives are quite costly and pose a potential hazard in storing and when used in operating equipment. Therefore, the oxidizer is added since it can oxidize NO to other NO x , which are more soluble, as well as avoid using chemical additives.
The modified process for removing NO x from the gas stream compromises of the following steps:
-
1.
Mixing the flue gas with air and feeding it into the oxidizer,
-
2.
Feeding the effluent gas from the oxidizer into the first scrubber where it comes in contact with an aqueous alkali solution,
-
3.
Feeding the gas stream from the first scrubber into the second scrubber where it comes in contact with the recycled alkali solution from first scrubber.
In the first scrubber, the gas mixture is treated by an alkali solution of higher concentration (15–20% NaOH) and in the second scrubber with a weaker solution (8–10% NaOH). In both columns, an intensive circulation of the absorption solution is used in order to reach a high intensity of mass transfer. The oxidizer is needed prior to scrubbing to oxidize NO to NO2 since NO is insoluble in water and in alkali solutions.
Prior to entering the oxidizer, the flue gas stream 1 (Fig. 1) which is mainly composed of NO x is mixed with air so that the desired O2/NO ratio is achieved. The oxidation reaction is normally very slow. Therefore, a large hollow vessel oxidizer is needed to maintain the necessary residence time for the gas mixture to reach a 50% conversion of NO to NO2 (reaction 1). The oxidizer is equipped with an internal heat exchanger (with large surface area) to control the temperature (below 200°C) given that reaction (2) is highly exothermic and NO2 will convert back to NO at a temperature above 200°C. Reactions (3) and (4) are very fast and the equilibrium state is reached in 10.2–10.3 s. Both reactions are highly exothermic and thus they are commonly carried out at low temperatures and high pressures. At atmospheric pressure and relatively low temperatures, the concentrations of both N2O3 and N2O4 are very low (no more than several percent); however, the concentration of N2O3 is always lower than N2O4. The second step of the process is the absorption of the NO x and N2O x by the alkali solution. The gases from the oxidizer are fed into the first scrubber wherein the alkali solution is added to the first scrubber to maintain the oxidative conditions. The second scrubber will remove the remaining NO x and N2O x from the gas stream. The concentration of the alkali solution is about 15 to 20% by weight.
Process kinetics and equilibrium
The NO oxidation to NO2 is a rate limited third-order gaseous reaction and the rate equation is (Lietti 1996):
The integrated form of the above Eq. 1 (Thomas and Vanderschuren 2000) is:
where k is the rate constant mol l−1 s−1; a a half of the initial concentration of NO, mole fraction; χ initial concentration of O2, mole fraction; α degree of oxidation of NO; τ residence time, s; and P pressure, atm
The values of the rate constant, k, for temperatures range from 0 to 390°C were statistically processed with Stat View statistic software and the following equation was obtained:
The formation N2O3 from NO and NO2 via reaction (3) and N2O4 formation (NO2 dimerization) via reaction (4) are limited by chemical equilibrium. The equilibrium composition of the gas mixture when both reactions occur simultaneously was calculated using EQ4WIN software. The data obtained for different temperatures was processed with Stat View and the following equations for the dependence of the equilibrium constants on temperature were obtained:
N2O3 formation
N2O4 formation
The alkali absorption rate was calculated by the following equation:
where y degree of absorption; k absorption rate constant mol l−1 s−1; q specific mass transfer area of the packing, m2/m3; and τ absorption time, s
For the required degree of absorption and the selected kind and size of packing, the absorption time was calculated.
Results and discussion
The optimum absorption rate of NO x by the alkali solution (NaOH) is attained at a NO to NO2 ratio of 1:1, which corresponds to the formation of N2O3 via reaction (3). Hence, an oxidation reactor (oxidizer) is installed ahead of the absorption column to ensure that an optimum degree of NO oxidation in the gaseous mixture is achieved before it enters the first scrubber (Fig. 1). The oxidation of NO to form NO2 proceeds according to the gaseous reaction (2). The equilibrium state depends strongly on temperature, pressure, and excess oxygen. If the reaction occurs under adiabatic conditions, a large quantity of heat is released and the temperature will increase significantly, depending on the concentration of NO in the gaseous mixture. For example, if NO content is 10% by volume and the degree of oxidation reaches 50%, the temperature will rise to about 100°C. At a temperature higher than 200°C, the reverse reaction of the NO oxidation (reaction 2) will be observed, i.e., NO2 decomposes to NO. On the other hand, low temperature, high pressure, and excess oxygen shift the equilibrium towards NO2. In addition, as stated above, reaction (2) is a third-order reaction, and therefore, depends strongly on NO and O2 concentrations. Therefore, the rate of reaction is very low at low NO concentrations. Reactions (3) and (4) are very fast and the equilibrium state is reached within 0.01 s. As these reactions are very fast, they do not affect the alkali absorption process. N2O3 and NO2 absorption by the alkaline solution (reactions 5 and 6) are irreversible. The degree of absorption depends mainly on the absorption rate constants and is nearly independent of the N2O3 and NO2 partial pressures.
In the alkali scrubber NO oxidation (reaction 2) as well as reactions (3) and (4), occur simultaneously with the absorption reactions (5) and (6). If both processes are to take place simultaneously, the total process rate is lower than when the oxidation and absorption occur consecutively. Consequently, in the modified process an oxidation reactor was included ahead of the absorption column so that the optimum degree of oxidation (50%) is attained. If an oxidation reactor is not included prior to the absorption column, then the NO oxidation will run solely in the absorption column(s). In this case, the packing of the absorption column must provide enough space to ensure a sufficient residence time for the oxidation of NO. In the new design, the gas space in the absorbers is not of importance. Hence, less packing may be used, reducing the size of the absorbers. The modified scheme also includes an external heat exchanger to absorb heat that is generated from reactions (5) and (6) (highly exothermic) in the absorption column (Fig. 1). Table 1 shows a stoichiometric material balance based on reactions (1) through (6). The calculations are performed for 1 kg of scrap gold and for the capacity of the reactor (125 kg of scrap gold). In addition, average flow rates per hour based on a cycle time of 1.5 h are calculated and shown in Table 1. Table 2 shows the material balance calculation in the oxidizer with respect to only reaction (2) at several different O2:NO ratios. The O2:NO ratio in the oxidizer is controlled by the amount of air mixed with the waste gas prior to entering the oxidizer. In Table 3, the compositions of the various NO x at the outlet of the oxidizer are shown at a O2:NO ratio of 1 with respect to only reaction (2). The heat evolved by the reaction is also assumed to be completely removed, hence maintaining the temperature at 20°C.
Tables 4, 5, 6, and 7 show results from the material balance on the oxidizer where a basis of 125 kg scrap gold is assumed; the calculations are based on the degree of NO oxidation as well as the assumption that the equilibrium states of reactions (3) and (4) are attained. The quantities and compositions of the gaseous mixtures are shown in mass (kg), volume (m3), and molar units (kmol). All results in Tables 4, 5, 6, and 7 are calculated based on the optimal degree of NO oxidation (50%). As no data on the vacuum in this system is available, the absolute pressure in the whole waste gas treatment system is assumed to be 0.5 atm and the pressure drop is neglected. Tables 4, 5 and 6 show the quantities and compositions of the gaseous mixture after the oxidizer when the equilibrium state of reactions (3) and (4) is attained, given a basis of 125 kg scrap gold. The difference between these results is the assumed percentage of reaction heat removed and the respective temperature calculated after the oxidizer. Shown in Table 7 are some of the basic parameters of the oxidizer, calculated based on the results shown in Tables 4, 5, and 6. The oxidizer volume is calculated for the three cases with different percentage removals of reaction heat by cooling water. The calculations are based on the average gas flowrate. The results in Table 7 show that if the oxidizer is adiabatic, the size of the oxidizer will be unacceptable. On the contrary, if the reaction heat is completely removed, the reactor size is significantly smaller. Yet this decrease in size due to heat removal will have an adverse effect on the internal heat exchanger area required, therefore the optimum choice should be economically based. In addition, some specific characteristics of the existing system could be decisive factors, e.g., system arrangement, fan duty, etc.
In Table 8, the quantity and composition of the treated waste gas after scrubbing is shown. The calculations are based on the assumption that the residual concentration of NO x (recalculated as NO2) after the second absorber is decreased down to the recommended limit of 500 mg/m3 (250 ppm).
Table 9 shows the oxidation reactor feed (stream 3) and effluent (stream 4) composition per 125 kg scrap gold at 50% NO oxidation in the reactor. It is assumed that the equilibrium states of reactions (3) and (4) are attained. Excess air was fed to the reactor (O2:NO = 1) with the reaction heat completely removed. The process was carried out at a constant temperature (20°C) and pressure (0.5 atm). The heats of the reaction were removed completely by cooling water. The composition of the final effluent (stream 12) after the second scrubber is also shown. The calculations are based on the assumption that the residual concentration of NO x (recalculated as NO2) after the second absorber is decreased down to the recommended limit of 500 mg/m3 (250 ppm). To meet this requirement, the total degree of absorption must be equal to or greater than 99.9% (Table 10). Based on the size and other parameters of the existing scrubber, the residence time was calculated to be 13.2 s per cycle using Eq. 6. An average value of the absorption rate constant was used (Thomas and Vanderschuren 2000). The optimum distribution of the absorbed N2O3 between the two scrubbers may be calculated based on the real data available on the packing and sizes of both columns. Table 11 shows a few basic parameters of the oxidation reactor that are calculated on the basis of the results. The calculations are based on the average gas flowrate.
The basic flowchart of the modified treatment system of the waste gas is shown in Fig. 1. The modifications are based on the grounds of basic principles and experimental work. The waste gas needs to be mixed with air at a fixed ratio to ensure the optimum degree of NO oxidation. The gas mixture goes into the oxidizer, which is a hollow column with an internal heat exchanger (cooler). The oxidizer is designed to give the optimum degree of NO oxidation (about 50%). Emirates Gold refinery uses a batch process, but the waste gas treatment system is a continuous system. The oxidizer will play the role of an equalization unit and thus help dampen the fluctuations of the gas composition and temperature. The gas mixture from the reactor enters the first scrubber (existing) where most of the N2O x , mainly as N2O3, is absorbed by a 15–20% NaOH solution. The remaining N2O x , also mainly as N2O3, is removed from the gas in the second scrubber by an 8–10% NaOH solution. The mass transfer area of the scrubbers packing, the solution circulation rate and the cooling duty were selected in order to attain the desired degree of N2O3 and NO2 absorption to achieve the regulation residual value of NO x of 500 mg/m3 (250 ppm).
Detailed design specifications for the waste gas treatment system were performed using Aspen Tech simulation software (HYSYS, Fig. 2) as well as Super Pro designer (Fig. 3), in accordance with hand calculations. The units were manufactured and added to the existing treatment system. Samples were taken by Emirate Gold DMCC chemist and Dubai municipality personnel, where both parties found that the modified and improved process meets the regulation standards.
Conclusion
The above recommendations were considered by the Emirates Gold DMCC administrators in order to meet the UAE air emission regulations. Based on the analysis of environmental laboratory of Dubai municipality, Emirates Gold DMCC were able to meet the required UAE regulations for NO x emission. It was also found that using an oxidizer prior to the scrubbing system, required a smaller number of scrubbers in comparison to the conventional method of a treatment system consisting only of scrubbers.
References
Barnes JM, Apel WA, Barrett KB (1995) Removal of nitrogen oxides from gas streams using biofiltration. J Hazard Mater 41(2–3):315–326
Chien T, Chu H (2000) Removal of SO2 and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution. J Hazard Mater B80:43–57
Chironna R, Altshuler B (2009) Chemical aspects of NO x scrubbing. Environmental Center. http://www.environmental-center.com/articles/article1011/article1011.htm. Accessed 23 March 2010
Coates TJ (1987) Absorption from air streams using aqueous solutions. AIChE Summer National Meeting, Technical Paper 9C
du Plessis CA, Kinney KA, Schroeder ED, Chang DPY, Scow KM (1998) Denitrification and nitric oxide reduction in an aerobic toluene-treating biofilter. Biotechnol Bioeng 58(4):408–415
Dubai Municipality (2008) Air quality monitoring in Dubai. Government of Dubai. https://portal.dm.gov.ae/AirQuality/AirqualityStandards.htm. Accessed 12 Apr 2010
Economidis N, Coil R, Smirniotis P (1998) Catalytic performance of Al2O3/SiO2/TiO2 loaded with V2O5 for the selective catalytic reduction of NO x with ammonia. Catal Today 40(1):27–37
Flanagan WP, Apel WA, Barnes JM, Lee BD (2002) Development of gas phase bioreactors for the removal of nitrogen oxides from synthetic flue gas streams. Fuel 81:1953–1961
Günther L (2010) NO x separation from drawn off air: a method of recovery of a high-quality substance from drawn off air without any waste formation. DGE- Nox-Entfernung aus Abluft. http://www.dge-wittenberg.de/english/produkte/waescherprogramm/nox.html. Accessed 20 April 2010
Heidenreich S, Nacken M, Hackel M, Schaub G (2008) Catalytic filter elements for combined particle separation and nitrogen oxides removal from gas streams. Powder Technol 180:86–90
Lietti L (1996) Reactivity of V2O5&z.sbnd;WO3TiO2 de-NO x catalysts by transient methods. Appl Catal B 10(4):281–297
Massucci M, Clegg SL, Brimblecombe P (1999) Equilibrium partial pressures, thermodynamic properties of aqueous and solid phases, and Cl2 production from aqueous HCl and HNO3 and their mixtures. J Phys Chem A 103(21):4209–4226
Patwardhan JA, Joshi JB (2004) Unified model for NO x absorption in aqueous alkaline and dilute acidic solutions. AIChE J 49(11):2728–2748
Qian Z et al (2010) Seasonal pattern of the acute mortality effects of air pollution. J Air Waste Manag Assoc 60:481–488
Radojevic M (1998) Reduction of nitrogen oxides in flue gases. Environ Pollut 102(1):685–689
Thomas D, Vanderschuren J (1996) The absorption–oxidation of NO x with hydrogen peroxide for the treatment of tail gases. Chem Eng Sci 51(11):2649–2654
Thomas D, Vanderschuren J (2000) Nitrogen oxides scrubbing with alkaline solutions. Chem Eng Technol 23(5):449–455
Wallin M, Karlsson CJ, Skoglundh M, Anders P (2003) Selective catalytic reduction of NO x with NH3 over H-ZSM-5: Influence of transient ammonia supply. J Catal 218:354–364
Acknowledgments
The authors are grateful to Mr. Mohamed Shekarchy, Director of Emirates Gold DMCC, and Engineer Faisal Dawood for their continuous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aidan, A., Alnaizy, R., Nenov, V. et al. Process design of waste gas treatment from Emirates Gold Refinery. Clean Techn Environ Policy 13, 447–457 (2011). https://doi.org/10.1007/s10098-010-0323-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-010-0323-3