Abstract
Generation of solid waste is inherent to manufacture of leather from skin and hide. Solid wastes generated at various unit operations of the tanning process considerably vary in quantity and composition. Fleshing is a type of animal tissue waste generated during the preparatory leather processing stage in relatively larger quantities as compared to other types of solid waste in the tanning industry. Fleshing mainly contains fat and protein and residual chemicals such as lime and sulphide used in the ‘unhairing’ process of beam house operation. Another type of solid waste in tanning industry which requires safe disposal is the primary sludge from tannery wastewater treatment plant. This study shows that both fleshing and primary sludge contains a significant quantity of volatile solids amenable for biodegradation. Different proportions of waste fleshing and primary sludge were subjected to anaerobic digestion. The studies were carried out in a laboratory scale reactor with an aim of developing an appropriate technology for recovery of bioenergy from the waste and subsequently ensure their safe disposal. Volatile solid destruction between 41 and 52%, specific gas production between 0.419 and 0.635 l/g volatile solids feed and methane yield between 71 and 77% were achieved. Further, the biomethanation potential of animal fleshing and substrate specific kinetics of the reaction process were also examined.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial processes generate waste varying in composition and quantity at various stages, and disposal of such waste has become more and more difficult, particularly in view of stringent environmental pollution control standards stipulated by statutory authorities. With rapid depletion of conventional energy sources, the need to find an alternative, preferably renewable, source of energy from waste is becoming increasingly important for the sustainable development (El-Mashad et al. 2004). Leather processing in tannery industries produces huge amount of both solid and liquid wastes. About 800 tonnes per day (tpd) of solid waste and 50 million litres per day (MLD) of liquid waste are being generated from tannery industries in India. It has been observed that 60–65% of the waste generated is predominantly organic and putrescible in nature (Saravanabhavan et al. 2004).
It is important to evaluate appropriate techniques and/or technologies for effective energy recovery from waste. Anaerobic digestion is considered as the one of the best treatment method for organic fraction of the segregated waste. Anaerobic digestion technologies ensure recovery of energy in the form of biogas, which is a clean fuel as compared to other conventional solid or liquid fuels. Among the many biological treatment methods experimented so far, anaerobic digestion possesses several advantages such as low energy requirement, low sludge production, low nutrient requirements and possibility of operation at high organic loading rate at a relatively low hydraulic retention time (Oliveira et al. 2004).
Scope
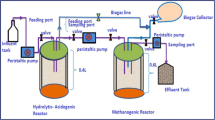
In order to examine appropriate technologies for the management of solid waste from tanning industry, anaerobic digestion process was studied using bench top reactors (serum bottles). Batch studies were performed with limed fleshing, an animal tissue waste obtained in the preparatory stages of leather processing in a tannery and primary sludge from the tannery effluent treatment plant. Anaerobic digestion was carried out under mesophilic condition. Batch reactors were operated at different initial volatile solid concentrations with a retention time (RT) of 8 weeks. Further, the effect of volatile solids concentration on biogas generation was evaluated.
Process parameters
Some of the commonly used anaerobic process indicators include pH, chemical oxygen demand (COD) destruction, volatile solid (VS) destruction, volatile fatty acid (VFA) concentration, gas production and gas composition. Kinetic studies on raw domestic primary sludge have reported the effectiveness of an acid phase process in destruction of COD of the substrates (Held et al. 2002). Most of the indicators listed above have been reported as appropriate for evaluating the effect of organic loading rate on the bioconversion of organic substrates and hence, these parameters have been chosen for assessing the decomposition of the substrate investigated in this study.
Experimental
Experimental setup
A simple methanogenic activity test procedure was adopted (Isa et al. 1993; Jawed and Tare 1996). The composition of limed fleshing, primary sludge and inoculum used in the batch experiments has been described in Table 1. A known amount of substrate containing a mixture of waste was transferred into serum bottles (with working volume of 70, 90, 110 and 130 ml). Appropriate quantities of waste materials were mixed and added to the serum bottle to obtain an initial VS load in the range of 1.5–3.5 g in all the reactors (Table 2).
As the substrates were being sourced from animal tissue, no additional nutrients were required to be supplemented to enhance the growth of biomass during the test period (Soto et al. 1993; Coates et al. 1996). The total gas production was measured using a liquid displacement method at an interval of 24 h after 3–5 days of start-up period. Contents of the serum bottle were mixed manually, after every gas measurement. Daily gas production was recorded. The entire test was conducted at a temperature of 30 ± 3°C for a period of 8 weeks.
Preparation of substrate
The limed fleshing used as substrate was grounded to less than 6-mm diameter using a meat-grinding machine. To this, primary sludge from the wastewater treatment plant of a tannery was added as diluent in the ratio of 1:1 (w/w basis) to maintain the fleshing solids in suspension and to achieve the desired level of flowability in the feed mixture. Primary sludge would also act as a source of various microorganisms required for anaerobic digestion process.
Inoculum
Pre-digested material consisting of all the essential microbes (hydrolyzing, fermentative, acetogenic and methanogenic bacterial consortium) has been chosen as an inoculum for the study. The inoculum was synthesized in the laboratory using cow dung, limed fleshing and primary sludge in equal weight. After cessation of gas production, the pre-digested material was tested for its activity with known quantity of sodium acetate as substrate for gas production, and later the digested residue was used as inoculum for the study.
Analyses
Total solids (TS), Volatile solids (VS), and Volatile Fatty Acids (VFA) were estimated according to the procedures recommended in the Standard methods for examination of water and waste water (APHA 1998). Assay bottles were periodically analysed for the above-mentioned parameters for a period of 8 weeks. Gas production from the reactors was monitored by means of water displacement method on a daily basis. The volume of water displaced from the bottle was equivalent to the volume of gas generated at the temperature and pressure that prevailed during the study period. The total methane content present in the gas was evaluated by alkali scrubbing method—a known quantity of gas drawn out using a sterile syringe was injected back into a liquid displacement system containing strong potassium hydroxide solution and the volume of methane gas present in biogas mixture was determined by the volume of alkali displaced against the known quantity of biogas injected.
Microscopy
Optical (phase contrast and epifluroscence) microscopic techniques were performed by adopting standard procedures for sample preparation, observation and photographic documentation (Zellner et al. 1993). Total cell counts were estimated using an improved Neubaur chamber (Visser et al. 1991; McHugh et al. 2003).
Experimental procedures and sampling schedules
Measured quantity of both solid and liquid waste corresponding to each volatile organic load was added in eight bottles after evaluating the substrate composition. At the end of every week, one bottle for each VS load and its corresponding control bottle were analysed for the various parameters. Thus, test reactors for three different organic loading and the corresponding control (pre-digested sample) reactors were constructed.
Result and discussion
Microscopy
Figure 1 shows the epiflurosence photomicrograph exhibit of the pre-digested sample used as inoculum. The study demonstrates the use of fluorescent probes for determinative microscopy of methanogenic archaea (Stahl et al. 1995). The total methanogenic population present in pre digested sample was enumerated using a Neuabaur chamber (Chin and Janssen 2002). The same sample was plated on simple methanogenic medium under strict anaerobic condition and enumerated for methanogenic population. The sample was found to contain an average of 2.1 × 109 cells/ml. These counts illustrate that the active methanogenic biomass present in the pre-digested sample are sufficient for batch study (Chin and Janssen 2002).
Gas production
The cumulative gas production from each of the test reactors operating at various organic loading of tannery solid waste and primary sludge is shown in Fig. 2. A cumulative gas production from test reactor R1 (VS concentration of 17.2 g/l) was 648 ml at the end of the eighth week of the study period and the peak gas production of 34 ml was observed on the 32nd day. Gas production almost ceased during the eighth week. A cumulative gas production from the test reactor R2 (VS concentration of 21.2 g/l) was 1,484 ml and a peak gas production of 133 ml was observed on 35th day while a cumulative gas production of 1860 ml was observed in test reactor R3 (VS concentration of 26.7 g/l) with the peak gas production of 150 ml observed on the 46th day. A gradual shift was observed in the period of peak gas production with increasing initial VS concentration. Figures 3, 4 and 5 illustrate the variation of VS destruction and cumulative gas production in the three reactors R1, R2 and R3, respectively. Specific gas production in terms of volatile solids fed ranged between 0.419 and 0.635 l/g VSin. Methane content in the biogas generated in the test reactors is shown in Table 3. The specific gas production and biogas composition obtained was comparable with the trends reported in published literature (Ramanujam et al. 2002; Gavala et al. 2003). Figures 6 and 7 show the logarithmic plot of gas production and time and daily gas production observed in the reactor, R1.
VS destruction in batch reactors
During anaerobic digestion of solid waste, biogas generation is more specifically related to reduction of biodegradable fraction of VS in the digester. The variation of VS reduction and the cumulative gas production with respect to time are shown in Figures 3, 4 and 5. Table 4 shows initial and final values of pH, VS concentration and VS reduction in the control and test reactors. VS reduction in the test reactors was observed in the range of 41–52%. These values are comparable with the VS reductions reported in the literature for various substrates (Ramanujam et al. 2002; Gavala et al. 2003; Song et al. 2004).
The values of VS destruction indicate that high initial VS concentration in the test reactors R2 and R3 affected the anaerobic digestion process and these reactors achieved much lesser VS destruction of 44.33 and 41.20%, respectively, when compared to test reactor R1, which achieved 51.97%.
Volatile fatty acid production in the batch reactors
The total VFA concentration in each test reactor is shown in Table 5. The maximum VFA concentration was observed during the second assay period in all the reactors, which indicates that the organic acid producers have demonstrated their activity during this period (Gavala et al. 2003). The variation of VFA with respect to time for each test reactor is shown in Fig. 8. It is observed that the concentration of volatile fatty acids present in the reactors at the end of the study period is in the range of 1440–1800 mg/l. The reduction in the concentration of VFA beyond second assay period and simultaneous increase in gas production beyond this period confirm the onset of methanogenic activity in the system during the second and the third week of the study period (Figs. 9, 10 and 11) (Ramanujam et al. 2002 and Gavala et al. 2003).
Biodegradability of feed mixture
The refractory fraction in the feed mixture is an indicator of the extent of biodegradability of the substrate. It is the portion of initial VS that remains in the digester as solid retention time (SRT) approaches infinity (Borja et al. 1995). The refractory fraction was determined graphically from the intercept of the plot drawn between (S/S 0) and (S 0 · HRT)−1 where S = substrate concentration (g/l), S 0 = initial VS concentration (g/l) and HRT = hydraulic retention time (d). The biodegradability of feed mixture was determined from the intercept. It was in the range of 34–43% of the influent volatile solids concentration (Table 6). The biodegradability factor indicates the presence of resistant volatile matter in the major portion of volatile solids in the digester. This reasonably conforms to the experimentally determined VS destruction efficiency of 41–52% in Table 6, (Parawira et al. 2004). Hence, it is essential to monitor the biodegradable fraction of VS in the feed to have better operational control over the process (Figs. 12, 13, 14).
Kinetic analysis
Anaerobic digestion process is generally described by first order kinetic model, which is based on the following two factors (Mahmoud et al. 2004; Parker 2005):
-
(1)
The rate of substrate conversion to biogas is directly proportional to the substrate concentration and
-
(2)
The volume of gas generated is proportional to the mass of the substrate destroyed
The corresponding kinetic equations are:
where S is the final substrate concentration (g/l); S 0 is the initial substrate concentration (g/l); k is the rate constant (d−1); t is the time (days); G is the cumulative gas production (l); V is the volume of reactor (l); C is the yield constant (l/g).
For a batch reactor the substrate remaining in the digester is given by integrating (1)
where t 0 is the lag time (d).
This model (Fulford 1988; McCarty and Mosey 1991) describes the average reactor behaviour at a longer retention time. Substituting S from Eq. 3 in Eq. 2, the cumulative gas production can be predicted by
Rearranging by taking natural logarithm gives
Knowing the yield constant (C), plot of ln(−G/(CVS 0)) versus time gives slope of −kt and intercept of kt 0 from which the rate constant and lag time can be calculated. The yield constant, rate constant and ‘lag time’ for various initial VS concentrations are given in Table 7. The onset of maximum gas generation was observed more or less in the same period as predicted by using Eq. 5. An example of the graphical analysis of the data for the reactor R1 is shown in Figs. 10 and 11.
Conclusion
The studies were carried out in a laboratory scale reactor with an aim of developing an appropriate technology for recovery of bioenergy from the waste and subsequently ensure their safe disposal. This study showed that both fleshing and primary sludge contains a significant quantity of volatile solids amenable for biodegradation.
The performance of anaerobic co-digestion tannery waste has been evaluated in terms of VS destruction, biogas yield and methane content. Based on the experimental data, the following conclusions have been drawn in support of biomethanation potential of the selected solid waste and substrate specific kinetics of the process.
As the inoculum was acclimatized with the substrates taken for the study and sufficient active biomass was present in the inoculum the start-up of the reactor has been achieved easily. It is confirmed, based on VS destruction efficiencies and specific gas production in terms of quantity of VS fed, that fleshing and primary sludge are amenable for anaerobic treatment for the recovery of biogas with high methane content. The batch reactors were operated with initial VS concentrations of 17.2, 21.2 and 26.7 g/l, and the corresponding specific gas production obtained in terms of volatile solids fed was 0.419, 0.635 and 0.535 l/g VSin, respectively. The VS destruction efficiency was 51.97, 44.33 and 41.19%, respectively. Methane content in the biogas varied between 71 and 77%.
The maximum VFA production during first 2 weeks indicates that the organic acid producers in the inoculum were sufficiently active. The rapid consumption of VFA observed in the subsequent weeks confirms the adequacy of methanogenic activity of methanogens present in the reactor.
Kinetic analysis of the data reveals that first order kinetic model is adequate to describe the anaerobic co-digestion of animal tissue and primary sludge. Larger the refractory fraction present in the feed mixture, longer the lag time for onset of biogas generation. The ranges of lag time obtained from the Eq. 5 for various initial VS concentrations are comparable with the experimentally observed time period of onset of maximum gas generation.
The composition of substrate indicates that the major constituents of volatile solids of the feed mixture are fats and proteins. Hence, the efficiency of anaerobic co-digestion of limed fleshing and primary sludge is dependent on the biodegradability of the types of fats and proteins present in the substrate. Hence, future studies should attempt to explore the impact of the fat and protein constituents of the tannery waste obtained in this study on its anaerobic digestion to gain better process control.
References
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington DC
Borja R et al (1995) Kinetic evaluation of an anaerobic fluidised-bed reactor treating slaughterhouse wastewater. Bioresour Technol 52:163–167
Chin K-J, Janssen PH (2002) Propionate formation by Opitutus terrae in pure culture and in mixed culture with a hydrogenotrophic methanogen and implications for carbon fluxes in anoxic rice paddy soil. Appl Environ Microbiol 68:2089–2092
Coates JD et al. (1996) Simple method for the measurement of the hydrogenotrophic methanogenic activity of anaerobic sludges. J Microbiol Methods 26:237–246
El-Mashad HM et al (2004) Design of a solar thermophilic anaerobic reactor for small farms. Biosyst Eng 87:345–353
Fulford D (1988) Running a biogas programme—a handbook, Appendix IV, Model for biogas digestion. Intermediate Technology Publication, London, pp 151–159
Gavala HN et al (2003) Mesophilic and thermophilic anaerobic digestion of primary and secondary sludge. Effect of pre-treatment at elevated temperature. Water Res 37:4561–4572
Held C et al (2002) Two-stage anaerobic fermentation of organic waste in CSTR and UFAF-reactors. Bioresour Technol 81:19–24
Isa MH et al (1993) Methanogenic activity test for study of anaerobic process. Indian J Environ Health 35(1):1–8
Jawed M, Tare V (1996) Methanogenic activity and performance of UASB, DSFF and USFF reactors. Water Sci Technol 34:483–487
Mahmoud N et al (2004) Anaerobic stabilisation and conversion of biopolymers in primary sludge-effect of temperature and sludge retention time. Water Res 38:983–991
McCarty PL, Mosey FE (1991) Modeling of anaerobic digestion processes (a discussion of concepts). Water Sci Technol 24:17–33
McHugh S et al (2003) Methanogenic population structure in a variety of anaerobic bioreactors. FEMS Microbiol Lett 219:297–304
Oliveira SVWB et al (2004) Formaldehyde degradation in an anaerobic packed-bed bioreactor. Water Res 38:1685–1694
Parawira W et al (2004) Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew Energy 29:1811–1823
Parker WJ (2005) Application of the ADM1 model to advanced anaerobic digestion. Bioresour Technol 96:1832–1842
Ramanujam RA et al (2002) Biomethanation—Technological Amenability for sustainable solid waste management in tanneries, 36th LERIG
Saravanabhavan S et al (2004) Natural leathers from natural materials: progressing toward a new arena in leather processing. Environ Sci Technol 38:871–879
Song YC et al (2004) Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic and thermophilic digestion of sewage sludge. Water Res 38:1653–1662
Soto M et al (1993) Methanogenic and non-methanogenic activity test. Theoretical basis and experimental set-up. Water Res 27:1361–1376
Stahl DA (1995) Use of fluorescent probes for determinative microscopy of methanogenic archaea. In: Sowers KR, Schreier HJ (eds) Archaea: a laboratory manual. Methanogens. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 111–121
Visser FA et al (1991) Diversity and population dynamics of methanogenic bacteria in a granular consortium. Appl Environ Microbiol 57:1728–1734
Zellner G et al (1993) Biofilm formation on polypropylene during start-up of anaerobic fixed-bed reactors. Biofouling 6:345–361
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thangamani, A., Rajakumar, S. & Ramanujam, R.A. Anaerobic co-digestion of hazardous tannery solid waste and primary sludge: biodegradation kinetics and metabolite analysis. Clean Techn Environ Policy 12, 517–524 (2010). https://doi.org/10.1007/s10098-009-0256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-009-0256-x