Abstract
To illustrate the effectiveness of our intensive multidisciplinary management (IMM) in the treatment of severely ill patients with necrotizing soft tissue infections (NSTIs). A retrospective observational study was conducted in a general ICU. Thirty-two consecutive patients undergoing IMM were carefully compared with 30 consecutive patients receiving a standard management (SM). IMM combined intensive care management, early surgical debridement followed by daily inspection of surgical wounds, close microbiological surveillance, and targeted high-dose antibiotics. IMM was associated with the better decrease of daily SOFA score (p = 0.04). Also, IMM caused + 12% increase in the overall number of surgical procedures (p = 0.022) and a higher number of tissue biopsies/per day (median 0.63 versus 0.32; p = 0.025), leading to a more targeted antimicrobial changes (89.6% vs 51.6%; p < 0.00001). High-dose daptomycin (75% vs 36.7%; p = 0.002) and extended/continuous infusion of beta-lactams (75% vs 43.3%; p = 0.011) were more frequently utilized. A specific efficiency score correlated with the decrease of SOFA score (efficacy) in IMM patients only (p = 0.027). Finally, IMM was associated with a significant lower ICU mortality rate (15.6% vs 40%; p = 0.032). IMM was more effective than SM as it allowed the earlier control of infection and the faster reduction of multiple organ-dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Necrotizing soft tissue infections (NSTIs) are uncommon severe bacterial infections characterized by high morbidity and mortality rate [1, 2]. Several studies report both the timing and adequacy of initial debridement as important determinants of survival [3,4,5,6,7,8]. Serial debridements are also highly recommended [9,10,11,12,13], but a recent survey reports that a “second look” surgery is performed in less than half of patients in the ICUs [14]. Moreover, the use of a multidisciplinary approach that associates intensive care management, rigorous and methodical surgical treatment, close microbiological surveillance, and prompt antibiotic therapy is logical although it often remains speculative. Since 2013 we implemented a task force including intensive care physicians, surgeons, microbiologist, infectious disease specialist, and clinical pharmacologist to offer the best-integrated approach for severe NSTI. Besides the standard treatment that includes intensive care support and early surgical debridement, our approach adds the daily inspection and medication of the open wounds in the operating room with regular fluids and tissue sampling for cultures. This approach aims to obtain the faster cleaning of the wounds and the closest microbiological surveillance. Furthermore, the regular sharing of microbiological results among intensive care physician, infectious disease consultant, microbiologist, and clinical pharmacologist allows for the most accurate antibiotic regimens and doses. The aim of this observational, retrospective study is to illustrate the most relevant aspects of our intensive multidisciplinary approach with respect to our previous “standard” of treatment.
Methods
Patients
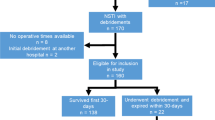
A retrospective observational study was conducted in the 8-bed general ICU of the Niguarda-Ca' Granda Hospital. A total of 62 consecutive patients were extracted from our electronic database since its very beginning in 2003 and divided in two groups. Thirty-two NSTI patients treated in accordance with our intensive multidisciplinary management (IMM) from January 2013 to December 2016 were compared with 30 consecutive patients submitted to our previous “standard” management (SM) from March 2003 to December 2012. As NSTI patients were centralized in our hospital only after 2010, a backward 10-year time interval was needed to make the comparison reliable. Both the medical ICU staff and the involved team of specialists remained unchanged from 2003 up today so assuring the constancy of clinical expertise and care. Patients were included if NSTI was diagnosed by CT scan imaging [2] and confirmed by presence of fascial edema and necrosis at the surgical inspection.
Data collection
Clinical, laboratory and demographic data, co-morbidities at admission, sequential organ failure score (SOFA) [15], simplified acute physiology score II (SAPS II) [16], ICU length of stay, and outcome were recorded. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was calculated for each patient [17].
NSTI data
The severity of NSTI was assessed by recording both the site and the extension of infection. A semi-quantitative score estimating the superficial extension of NSTI was created (Fig. 1). The score value aggregates either the surface area and the site of NSTIs. The score was validated by comparison with the worldwide used “Wallace’s rule of nines” that allows for quantification of burned skin extension [18] (Spearman’s rank test 0.87; p < 0.0001).
The microbiological agents responsible for NSTI were identified by blood cultures, open tissue biopsies, and fluid culture results. The ratio between the patient’s open tissue biopsies and ICU length of stay was calculated as index of intensive microbiological surveillance. The number of infectious disease specialist consultations, the antimicrobial regimen used, the changes of antibiotic therapy either empirical or guided by culture results, the use of high-dose daptomycin, and continuous infusion beta-lactams were reported.
The promptness of surgical treatment was assessed by the time interval between the onset of signs and symptoms and the first debridement. Also, the total time spent in OR during the first week was computed. Finally, the total of necrosectomies, amputations, and surgical medications in OR were recorded. The need for vacuum-assisted closure (VAC) therapy and hyperbaric oxygen therapy (HBOT) was also recorded.
Intensive multidisciplinary management
The IMM approach relies upon the synchronized and coordinated work of ICU physicians, emergency surgeons, infectious disease consultant, microbiologist, and clinical pharmacologist.
The main features of the surgical strategy included the daily medication of the infection site in OR and the methodical fluids and tissue sampling for cultures.
The initial antibiotic regimen included high-dose daptomycin plus meropenem or piperacillin-tazobactam. Clindamycin was added to inhibit microbial toxin production by group A Streptococci or Clostridia species. Daptomycin was continued until hemodynamic stabilization if Gram-positive results and refractory shock (norepinephrine dose > 0.3 μg/kg/min) were present. Extended or continuous beta-lactams infusion was employed to achieve constant drug concentrations of about 60–70% T > 4–5 × MIC [19,20,21,22]. Maintenance doses were often set empirically as therapeutic drug monitoring (TDM) could rarely be obtained. Full doses were maintained whenever generalized soft tissue edema and/or fluids losses ≥ 30 ml/h/m2 (≈ 1000–1200 ml/day) were present. Creatinine clearance < 70 ml/min/m2, low cardiac output state (< 2.2 l/min/m2), or daily increase of serum creatinine phosphokinase (CPK) > 500 UI or > 20% from baseline values > 5000 UI prompted the adjustment of the antibiotic dosage.
Standard management
At variance with IMM, the SM approach provided for the on-demand consultation only with the emergency surgeon and/or the infectious disease specialist. The surgeon was alerted only if purulent exudate, soft tissue ischemia, or necrosis were found or if severe sepsis or septic shock were ongoing. The infectious disease specialist was consulted only if multidrug-resistant (MDR) pathogens were isolated. The initial antibiotic regimen included vancomycin, ciprofloxacin, or broad-spectrum beta-lactams plus clindamycin (group A Streptococci or Clostridia). Daptomycin (6 mg/kg/day) was administered as second-line agent or as “rescue” therapy in more severely ill patients. The most relevant differences between the IMM and SM approach are resumed in Fig. 2.
Flowchart illustrating the most relevant differences between the IMM and SM approach. Abbreviations: LD loading dose, MD maintaining dose, tid ter in die, qid quater in die, EI extended infusion, CI continuous infusion, PK/PD pharmacokinetic/pharmacodynamic, OR operating room, MDR multidrug resistant, ICU intensive care unit
IMM quantification
To evaluate the impact of the interactive cooperation of IMM on the treatment of NSTI, an aggregate index was built that joined the efficiency of surgical treatment, microbiological surveillance, and targeting of the antimicrobial therapy during the first 7 days.
-
The efficiency of surgical treatment (Nsurg) was quantified by the sum of debridements + medications in OR.
-
The efficiency of antimicrobial therapy was assessed by the sum of antimicrobial therapy changes (∑changeATB: + 1 point for de-escalation, escalation or even targeted on culture results, − 1 point for empirical changes) plus pharmacokinetic (pk) optimization (0 absent; 1 present) weighted by the number of surgical procedures with tissue biopsies (NSURGbiopsies). Therefore:
Results were normalized by the number of days spent in ICU during the first week (n/7).
The efficacy of treatment was measured by the difference between the final and initial SOFA values during the first week.
A negative ΔSOFA reflected the patient’s improvement while a positive difference meant his worsening. The correlation between efficiency (x-axis) and efficacy (y-axis) was assessed by a scatterplot for either IMM and SM.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) or median and inter-quartile range (IQR) according to data distribution. The Student’s t test and the Mann-Whitney test were used for comparisons. Categorical variables were expressed as count or percentages and the Chi-square test or the Fisher’s exact test were used as appropriate. The individual values of daily SOFA score were averaged during the first 7 days thus obtaining the time course of SOFA for both IMM and SM. Also, the area under curve (AUC) of SOFA was calculated for each patient and normalized for the ICU stay (adjAUC) so allowing for inter-group comparison. A regression line between the efficiency and efficacy score was drawn and the Pearson’s r value calculated. A p value of < 0.05 was considered significant.
Results
Baseline characteristics of the patients are shown in Table 1. NSTIs involved pelvis, perineum, or inferior extremities in 40 cases (64.5%). All patients were severely ill (SOFA score 8.3 ± 4.4 pts and SAPS II score 41.7 ± 17.2 pts) with both groups being equivalent in terms of age, gender, co-morbidities, site of infection, extension score, and severity of illness (Table 2). Continuous renal replacement therapy (CRRT) was performed more frequently with SM (p = 0.04) mainly as consequence of deteriorating conditions during the ICU stay.
Surgical treatment
The median interval between the onset of sign and symptoms and the surgical treatment was 9.5 h (IQR 5–19). IMM was associated with lesser surgical debridements but more medications in OR (48% and 33% of the time spent in OR respectively; p = 0.013), so giving + 12% increase in the overall number of surgical procedures (p = 0.022). Open wound biopsies increased from 0.32 biopsies/day (IQR 0.16–0.59) to 0.63 biopsies/day (IQR 0.36–0.83) (p = 0.025). The use of VAC therapy also increased with IMM (p = 0.027) (Table 2).
Microbiological diagnosis and surveillance
Microbiological features are presented in Table 3. The initial tissue biopsies and fluid specimens were positive in 93.5% of cases, four patients only having negative cultures. Group A streptococci were isolated in 10 patients (mean SOFA score 11 pts). Anaerobes could be isolated in 12 patients only but gas collection in the deep fascia was present in other 19 patients. The closer microbiological surveillance by IMM allowed for the better adjustment of the initial antibiotic regimen with lesser (48 vs 62) but more targeted changes (43 vs 32; p < 0.00001). Empirical changes decreased dramatically from 30 to 5 only (p < 0.00001) (Table 2).
Antibiotic and adjuvant therapies
Both high dose daptomycin (p = 0.002) and extended or continuous infusion of beta-lactams (p = 0.011) increased with IMM (Table 2). No patients suffered from serious adverse effects in consequence of high-dose antibiotics. HBOT was performed as an emergency procedure in two patients with Clostridium spp. infection and as adjuvant to tissue repairing in other 12 patients.
Efficiency and efficacy
The efficiency profile differed markedly between IMM and SM the median value of the efficiency score being 5.25 pts. [IQR 1.64–9.5 pts] and 2.93 pts. [IQR 0.89–3.88 pts], respectively. High score values (> 5 pts) were recorded in 50% of IMM and 13.3% of SM patients (p < 0.01).
Efficacy also differed between groups. IMM was associated with a better evolution of the daily SOFA score (Fig. 3) and significantly lower adjAUC values (8 ± 5 pts. versus 11 ± 7; p = 0.04). A greater decrease of ΔSOFA (− 5.2 ± 3.5 pts. versus − 2.1 ± 3.0 pts., p = 0.003) was found in IMM patients. The scatterplot of the efficiency/efficacy relationship is shown in Fig. 4. A significant correlation was found with the IMM approach only (Pearson’s r − 0.39; p = 0.027).
ICU stay and survival
The median ICU length of stay was 8.5 days (IQR 3–16 days) with an overall ICU mortality of 27.4%. Almost all deaths followed refractory septic shock, the only two exceptions being intestinal infarction and lethal hyperkalemia. IMM was associated with a lower ICU mortality rate (15.6% vs. 40%; p = 0.032), the mortality at day 7th being already significantly different (IMM 3.1% vs SM 20%; p = 0.049).
Discussion
Severe NSTI requiring intensive care management is rarely encountered in clinical practice and needs for a coordinated multidisciplinary approach to achieve the best results [23]. To our knowledge, this is the first study showing that a coordinated and synchronized multidisciplinary strategy is the most effective in the management of severe NSTI in ICU.
In this small but very detailed population of severely ills [16], IMM allowed for the steeper decrease of the daily SOFA score as consequence of a better and earlier control of the infection.
The approach to NSTIs focuses upon three cornerstones: (1) timely and adequate surgical debridement [3,4,5,6,7,8,9,10], (2) strict microbiological surveillance, and (3) targeted high-dose antibiotic regimens. IMM added to these features the joined efforts of surgeons and ICU physicians to provide the day-by-day assessment of the open wounds thus helping in the preservation of demi-vital tissues. This was achieved by the regular insertion of additional procedures in the already very-busy schedule of our emergency OR. Also, the five-fold increase of VAC therapy reflects the care we used to keep the surgical wound as dry as possible in order to facilitate tissue granulation and healing.
The second cornerstone of IMM includes a strict microbiological surveillance. IMM patients underwent almost the double of tissue biopsies/day with respect to SM. This allowed for the more focused antibiotic strategy, the radical decrease of empirical antimicrobial changes, the increase of de-escalations, and the prompt identification and treatment of super-infections.
Daptomycin was used in reason of its high and quick bactericidal activity [24], larger diffusion in skin and soft tissues [25], and no toxin release in vitro from the infected cells [26]. High doses and continuous or extended infusion of beta-lactams [27] were employed to counteract the deleterious effects of exudative losses from the open wounds, permeability edema, tissue hypoperfusion, hypoalbuminemia [28, 29], glomerular hyperfiltration [30]. We also reasoned that high-dose antibiotics helped in avoiding underexposure levels in the first days of treatment so ensuring the greatest efficacy in the infection control. Nevertheless, no serious adverse effects from high antibiotic dosages were reported mainly as both organ function and CPK levels were carefully monitored.
We tried to quantify the synergistic effect of cooperation by creating a score that aggregates the efforts of all team-specialists. Our efficiency score correlated with the efficacy of treatment as independently measured by the ∆SOFA score. We are aware that the decrease of SOFA may occur in response to factors other than IMM (e.g., individual immune-inflammatory response to disease), but the correlation between efficiency and efficacy in IMM patients only supports the hypothesis of its superiority with respect to SM. Finally, IMM was associated with the reduction of mortality from 40 to 15.6%. We can only speculate about such reduction, although it is tempting to hypothesize that the faster and better control of the infection source contributed to the improved outcome.
Our study has several limitations. Firstly, it was retrospective and with a small number of patients enrolled. However, our population was very homogeneous as all patients were severely ill and in need for both cardiovascular and respiratory support (Table 1). This makes the comparison more reliable so supporting the hypothesis that IMM can help in the treatment of severe NSTI patients. Secondly, we created a semi-quantitative score to evaluate the extension of NSTIs. Although no validated scores are currently available for this purpose, its reliability was tested by comparison with the well-known “Wallace’s rule of nines” (Spearman’s rank test 0.87). Similarly, a new semi-quantitative score was created to evaluate the effectiveness of IMM. As no scores are presently available for comparison, no validation was possible. This score quantified the impact of elusive factors as cooperation and synchronization in the treatment of severe NSTIs. Nevertheless, it correlated with an objective and independent measure of disease severity as the SOFA score. So, IMM patients showed their clinical improvement (negative ∆SOFA values) in association with higher values of the efficiency score (Fig. 4). Finally, we did not routinely perform TDM in our patients so theoretically increasing the risk of toxic exposure to high-dose antibiotics. As the timely execution of TDM is rarely obtained in clinical practice, an empirical algorithm that combined careful clinical examination, serial assessment of patient’s hemodynamic status and regular biochemistry measurements was successfully used as a surrogate.
In conclusion, the combination of careful surgical strategy, close microbiological surveillance, and high-dose antibiotics allows for the faster and better control of infection with consequent reduction of organ damage. As this approach relies upon “good basic intensive care principles”, we believe it should be regarded as “standard of care” for any patient who requires surgery and sophisticated microbiological and antibiotics management.
References
Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, Lyon JL (2006) Cellulitis incidence in a defined population. Epidemiol Infect 134(2):293–299
Anaya DA, Dellinger EP (2007) Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 44(5):705–710
McHenry CR, Piotrowski JJ, Petrinic D, Malangoni MA (1995) Determinants of mortality for necrotizing soft-tissue infections. Ann Surg 221(5):558–565
Anaya DA, McMahon K, Nathens A, Sullivan SR, Foy H, Bulger E (2005) Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg 140:151–158
Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi-Benissan G, Hilbert G, Gruson D (2009) Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 35(5):847–853
Voros D, Pissitois C, Georgantas D, Katsaragakis S, Antoniou S, Papadimitriou J (1993) Role of early and extensive surgery in the treatment of severe necrotizing soft tissue infection. Br J Surg 80(9):1190–1191
Bilton BD, Zibari GB, McMillan RW, Aultman DF, Dunn G, McDonald JC (1998) Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: a retrospective study. Am Surg 64(5):397–400
Hadeed GJ, Smith J, O’Keeffe, Kulvatunyou N, Wynne JL, Joseph B, Friese RS, Wachtel TL, Rhee PM, El-Menyar A et al (2016) Early surgical intervention and its impact on patients presenting with necrotizing soft tissue infections: a single academic center experience. J Emerg Trauma Shock 9(1):22–27
Wong CH, Haw-Chong C, Shanker P, Khin LW, Tan JL, Low CO (2003) Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am 85(8):1454–1460
Elliot DC, Kufera JA, Myers RAM, Necrotizing soft tissue infections (1996) Risk factors for mortality and strategies for management. Ann Surg 224(5):672–683
Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC (2014) Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 59(2):e10–e52
Okoye O, Talving P, Lam L, Smith J, Teixeira PG, Inaba K, Koronakis N, Demetriades D (2013) Timing of redébridement after initial source control impacts survival in necrotizing soft tissue infection. Am Surg 79(10):1081–1085
Stevens DL, Bryant AE (2017) Necrotizing soft-tissue infections. N Engl J Med 377(23):2253–2265
de Prost N, Sbidian E, Chosidow O, Brun-Buisson C, Amathieu R (2015) Management of necrotizing soft tissue infections in the intensive care unit: results of an international survey. Intensive Care Med 41(8):1506–1508
Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 26(11):1793–1800
Le Gall JR, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA 270(24):2957–2963
Wong CH, Khin LW, Heng KS, Tan KC, Low CO (2004) The LRINEC (Labroatory risk indicator for necrotizing fasciitis) score: a tool for distinguishing necrotizing fasciitis form other soft tissue infections. Crit Care Med 32(7):1535–1541
Wallace AB (1951) The exposure treatment of burns. Lancet 1(6653):501–504
Roberts JA, Lipman J (2009) Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37(3):840–851
Abdul-Aziz MH, Lipman J, Roberts JA (2017) Identifying “at-risk” patients for sub-optimal beta-lactam exposure in critically ill patients with severe infections. Crit Care 21(1):283–285
Ulldemolins M, Roberts JA, Lipman J, Rello J (2011) Antibiotic dosing in multiple organ dysfunction syndrome. Chest 139(5):1210–1220
Blot S, Lipman J, Roberts DM, Roberts JA (2014) The influence of acute kidney injury on antimicrobial dosing in critically ill patients: are dose reductions always necessary? Diagn Microbiol Infect Dis 79(1):77–84
De Prost N, Lipman J, Mimoz O (2017) Therapeutic targets in necrotizing soft tissue infections. Intensive Care Med 43(11):1717–1719
D’Avolio A, Pensi D, Baietto L, Pacini G, Di Perri G, De Rosa FG (2016) Daptomycin pharmacokinetics and pharmacodynamics in septic and critically ill patients. Drugs 76(12):1161–1174
Kiang TKL, Häfeli UO, Ensom MHH (2014) A comprehensive review on the pharmacokinetics of antibiotics in interstitial fluid spaces in humans: implications on dosing and clinical pharmacokinetic monitoring. Clin Pharmacokinet 53(8):695–730
Hancock RE (2005) Mechanisms of action of newer antibiotics for Gram-positive pathogens. Lancet Infect Dis 5(4):209–218
Roberts J, Gavin MJ, Gordon Y, Choi S, Gomersall CD, Lipman J (2012) How to optimise antimicrobial prescriptions in the intensive care unit: principles of individualised dosing using pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents 39(3):187–192
Blot SI, Pea F, Lipman J (2014) The effect of pathophysiology on pharmacokinetics in the critically ill patient--concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11
Scaglione F (2015) Can we transfer pharmacokinetics/pharmacodynamics of antimicrobials into clinical practice? Int J Antimicrob Agents 46(1):S40–S42
Pea F (2016) Practical concept of pharmacokinetics/pharmacodynamics in the management of skin and soft tissue infections. Curr Opin Infect Dis 29(2):153–159
Acknowledgements
In memory of Giovanni Pietro Gesu, for his essential contribution in the implementation of a multidisciplinary task force between microbiologists, infectious disease specialists and intensive care physicians in Niguarda-Ca′ Granda Hospital. Special thanks are due to medical and nursing ICU staff for treating and caring the patient.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Professor Scaglione declares Personal Fees from Pfizer, Novartis, Bayer, and GSK. Other authors have no conflict of interest to declare.
Ethical standard statement
The study was performed in accordance with ethical standards laid down in the 1964 Declarations of Helsinki and its later amendments and with guidelines laid down by the hospital ethics committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 16 kb)
Rights and permissions
About this article
Cite this article
Gatti, M., Gasparini, L.E., Laratta, M. et al. Intensive multidisciplinary management in critical care patients affected by severe necrotizing soft tissue infections: a cooperative method to improve the efficacy of treatment. Eur J Clin Microbiol Infect Dis 38, 1153–1162 (2019). https://doi.org/10.1007/s10096-019-03521-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03521-2