Abstract
Gonorrhea is the second most frequently reported notifiable disease in the United States and is becoming increasingly common in Europe. The purpose of this review was to assess the current state of drug-resistant Neisseria gonorrhoeae in order to evaluate future prospects for its treatment. An exhaustive literature search was conducted to include the latest research regarding drug resistance and treatment guidelines for gonorrhea. Gonococci have acquired all known resistance mechanisms to all antimicrobials used for treatment. Currently, the European Union, the United States, and the United Kingdom have established surveillance programs to assess, on a yearly basis, the development of gonococcal resistance. Current treatment guidelines are being threatened by the increasing number of ceftriaxone-, cefixime-, and azithromycin-resistant N. gonorrhoeae strains being detected worldwide. This has led the scientific community to develop new treatment options with new molecules in order to persevere in the battle against this “superbug”.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gonorrhea is the second most frequently reported notifiable disease in the United States. In fact, about 820,000 new gonorrhea infections occur each year in the United States, of which an estimated 246,000 are resistant to at least one antibiotic (http://www.cdc.gov/drugresistance/biggest_threats.html). The latest report of the European Centre for Disease Prevention and Control (ECDC) showed a 25% increase in the number of reported cases between 2013 and 2014, with a total of 66,413 cases reported in 2014 (http://ecdc.europa.eu/en/healthtopics/gonorrhoea/Pages/Annual-Epidemiological-Report-2016.aspx). Seeing as there is no gonococcal vaccine, the public health control of gonorrhea relies entirely on prevention, sexual contact notification, epidemiological surveillance, diagnosis, and, especially, antibiotic treatment.
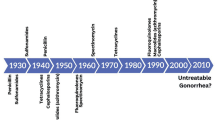
Since sulfonamides were first introduced to treat gonorrhea in the 1930s, Neisseria gonorrhoeae has continuously shown an extraordinary ability to develop resistance to all antimicrobials introduced for treatment [1] (Fig. 1). In fact, the recent emergence of resistance to the third-generation extended-spectrum cephalosporins (ESCs) cefixime and ceftriaxone, combined with resistance to practically all other available gonorrhea antimicrobials, has turned N. gonorrhoeae infection into a great public health issue [2]. So much so that, in 2012, the Centers for Disease Control and Prevention (CDC) classified it as a “Superbug”, alerting about the prospect of untreatable gonorrhea in the near future [3].
This review describes the latest discoveries regarding gonococcal resistance and future prospects for N. gonorrhoeae treatment.
Molecular mechanisms of drug resistance
Neisseria gonorrhoeae has proven to have the capacity to alter its DNA, due to the fact that it is naturally competent for transformation during its entire life cycle. Moreover, when exposed to selective pressure, it can also change its genome effectively through all types of mutations. In fact, studies [4] show that, after introducing a new antimicrobial drug, gonococci become resistant and replace sensitive bacterial population within two decades. In this way, gonococci have evolved and acquired or developed all known physiological resistance mechanisms to all antimicrobials used for treatment [5]: (i) enzymatic antimicrobial destruction or modification; (ii) target modification or protection reducing affinity for the antimicrobials; (iii) decreased influx of antimicrobials; and (iv) increased efflux of antimicrobials (Table 1).
Penicillinase is the main antimicrobial resistance determinant by which gonococcal strains decrease their susceptibility to antibiotics through their destruction or modification. Neisseria gonorrhoeae strains containing a plasmid with the bla TEM-1 or bla TEM-135 genes encode TEM-1 or TEM-135 β-lactamases, which hydrolyze the cyclic amide bond of β-lactamase-susceptible penicillins, opening the β-lactam ring, and, therefore, rendering it inactive [6, 7].
One of the main mechanisms of resistance in N. gonorrhoeae is target modification or reduction of target affinity. There are different genes that can alter antibiotic binding depending on the antimicrobial family. In this sense, alterations in the folP gene, which encodes the drug target DHPS for sulfonamides, is responsible for sulfonamide resistance [8]. The penA and ponA genes are responsible for chromosomally mediated β-lactam resistance due to specific mutations that modify the primary target PBP2 (penA) and the secondary target PBP1 (ponA), therefore reducing the penicillin acylation rate [6, 9, 10]. Similarly, tetracycline resistance is also due to two genes, rpsJ and tetM. The rpsJ gene produces resistance by encoding an altered form of a ribosomal protein, whereas tetM encodes a protein which binds to the ribosome, causing the release of the tetracycline molecule [11, 12]. Along these lines, the rpsE gene causes spectinomycin resistance by encoding an altered 30s ribosomal protein which disrupts the antimicrobial’s binding to 16s RNA [13, 14]. The main resistance mechanism for quinolones is also target modification. In this case, two genes are involved, gyrA and parC [15, 16]. Mutations in gyrA cause alterations in the primary target of DNA gyrase, which results in reduced quinolone binding affinity. As for parC, it is responsible for high-level resistance, as mutations in this gene affect the two parC subunits in topoisomerase IV. Finally, macrolide resistance also occurs due to this mechanism. In this case, a series of erm genes encode rRNA methylase, which produce resistance by blocking macrolide binding to 23s rRNA by methylating an adenosine residue at position 2058 [17].
Finally, N. gonorrhoeae can also develop antibiotic resistance by controlling the concentration of antibiotic entering the gonococci. Mutations in the genes porB1b [5, 10] and pilQ [8] produce alterations in porins, which result in a decreased influx of antibiotic into the cell. Similarly, overexpression of the pore-encoding mtrR [10, 16, 18] and mef [17] genes result in a higher number of efflux pumps, which decrease the concentration of antibiotic inside the cell.
Most gonococcal antimicrobial resistance (AMR) determinants have a chromosomal origin and only the bla TEM gene [12] and the tetM gene [19], which are responsible for high-level resistance to penicillin and tetracycline, respectively, are known to be plasmid-borne.
Generally, the acquisition of a single AMR determinant only results in an increase in the minimum inhibitory concentration (MIC) without it having any clinical importance. However, the cumulative effect of two or more AMR determinants and their interactions can ultimately result in clinical treatment failure [20]. Furthermore, some AMR determinants, such as mtrR and gyrA, are thought to enhance the fitness of some N. gonorrhoeae strains [16].
Current resistance status
As the CDC [2] predicted in 2012, N. gonorrhoeae has become a “Superbug” and, thus, an urgent public health threat. The increasing detection of gonococcal strains resistant to ESCs and azithromycin may lead to a situation where gonorrhea becomes untreatable. In fact, N. gonorrhoeae strains H041 (ceftriaxone MIC of 2 mg/L), F89 (ceftriaxone MIC of 1 mg/L), and A8806 (ceftriaxone MIC of 0.5 mg/L) with high-level resistance to ceftriaxone were isolated from patients in Japan [21], France [22], and Australia [23], respectively. Moreover, a new ceftriaxone- and multidrug-resistant N. gonorrhoeae strain was recently isolated in Japan [24]. This strain contained a novel mosaic penA allele encoding a new mosaic penicillin-binding protein 2 (PBP2) with an almost identical resistance-determining 3′-terminal region when compared to the same regions in strains previously reported in Australia and Japan.
Interestingly, another study recently published in Canada [25] showed that the proportion of isolates with decreased susceptibility to cephalosporins declined significantly between 2011 and 2014, whereas azithromycin resistance increased considerably during the same period. This study concluded that continued surveillance of gonococcal antimicrobial susceptibility is vital to modify treatment guidelines in order to slow down the spread of isolates with decreased susceptibility to cephalosporins and resistance to azithromycin.
The problem with resistant N. gonorrhoeae is such that the first case of failure of the standard dual antimicrobial treatment for gonorrhea was recently reported in the UK [26]. The results of the antimicrobial susceptibility tests showed that the strain was resistant to ceftriaxone, azithromycin, cefixime, cefotaxime, penicillin, tetracycline, and ciprofloxacin, but it was susceptible to spectinomycin.
As these reports show, treatment for gonococcal infection is being seriously threatened by the emergence of antimicrobial resistance. Bearing this in mind, governments all over the world have created programs to collect data in order to study the ways in which this species is developing resistances and presenting itself at a community level. Three of the most established programs are the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) in the United Kingdom (https://www.gov.uk/government/publications/gonococcal-resistance-to-antimicrobials-surveillance-programme-grasp-report), the Gonococcal Isolate Surveillance Project (GISP) in the United States (https://www.cdc.gov/std/gisp/), and the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP) (http://ecdc.europa.eu/en/healthtopics/gonorrhoea/response-plan/pages/strengthening-antimicrobial-surveillance.aspx). All three programs share a common aim: to detect the emergence of any AMR which could threaten the effectiveness of current first-line treatments.
Reports from all three of these programs provide insights as to how antibiotic resistance is evolving within N. gonorrhoeae strains. Figure 2a–c show said evolution for the 2004–2014 period. As these graphs illustrate, different treatment policies between the United Kingdom, the United States, and the European Union have resulted in different antibiotic resistance trends. For example, the GRASP reports a decrease in penicillin resistance from 2007 to 2011, followed by an increasing trend ever since, having reached 22.6% in 2014. Similarly, the GISP reports also show a consistently increasing trend in penicillin resistance having more than doubled during the last ten reports (from 16% in 2004 to 38% in 2014). On the other hand, the Euro-GASP shows a stable rate of penicillin resistance at around 13–14% since 2004. In regard to ceftriaxone, all three reports show resistance rates close to 0%. GRASP and Euro-GASP detected a peak in cefixime resistance in 2011 and 2010, respectively, after which resistance rates decreased considerably in both cases (11 to 1.4% for GRASP and 9 to 2% for Euro-GASP). The detection of ciprofloxacin-resistant strains increased in GRASP since 2012 (25 to 37.3% in 2014) and in GISP since 2009 (12 to 21% in 2014). On the other hand, Euro-GASP reports show a decrease in ciprofloxacin resistance from 63% in 2009 to 50% in 2014. Finally, azithromycin resistance varies considerably among reports. The GRASP shows rates of azithromycin resistance below 2% since 2007, whereas the GISP and the Euro-GASP show increasing detection of resistance in their 2014 reports (from 1 to 4% in GISP and from 5.4 to 7.9% for Euro-GASP).
Treatment options
In clinical practice, treatment for gonococcal infection is mostly given empirically at the first clinical visit; thus, antimicrobial susceptibility is rarely performed prior to prescription. According to the World Health Organization (WHO) guidelines [27], first-line antimicrobial therapy should be highly effective, widely available and affordable, lack toxicity, single dose, and (rapidly) cure at least >95% of infected patients. However, identical cut-off levels and treatment regimen(s) will not be the most cost-effective solution in all geographic regions and populations [28].
For the last decade, in many geographic regions worldwide, cefixime 400 mg orally or ceftriaxone 125–1000 mg intramuscularly or intravenously had been the recommended first-line monotherapy for gonorrhea [28, 29]. However, due to the previously commented emergence of resistance to all extended-spectrum cephalosporins (ESCs), including the most potent ESCs cefixime and ceftriaxone, dual antimicrobial therapy has been introduced as first-line empirical therapy for uncomplicated anogenital and pharyngeal gonorrhea in the USA [30], Canada [31], Australia [32], and Europe [33]. This treatment generally consists of a single 250–500 mg dose of ceftriaxone along with 1–2 g of azithromycin, which additionally eradicates concomitant Chlamydia trachomatis infection.
However, the decreased susceptibility to ceftriaxone and increased resistance to azithromycin worldwide suggest that the recently introduced dual antimicrobial regimens might not be effective long-term solutions. Two new dual antimicrobial therapies were recently proposed and evaluated: 240 mg of gentamicin intramuscularly plus 2 g of azithromycin orally and 320 mg of gemifloxacin orally plus 2 g of azithromycin orally [34]. The cure rate was 100 and 99.5%, respectively, which proves any of these two regimes suitable as alternative treatment options in the presence of ceftriaxone resistance, treatment failure with the recommended regimen, or ESC allergy [30]. Table 2 shows the suggested treatment guidelines by the Centers for Disease Control and Prevention (CDC), the European Union, and the WHO. Furthermore, many analogues of previously described and used antimicrobials have also been shown to have a high in vitro activity against Neisseria gonorrhoeae. These include several new fluoroquinolones [35–38], tetracyclines [39, 40], carbapenems [41], macrolides [42, 43], and the lipoglycopeptide dalbavancin [44]. The new oral fluoroketolide solithromycin [43] (macrolide family) is the most advanced in development, currently having a multicenter, open-label, randomized Phase 3 clinical trial running.
Concluding remarks
Gonorrhea is becoming an even bigger worldwide public health problem with the emergence of multidrug-resistant gonococcal strains. In order to avoid the scenario where gonococcal infections become untreatable, efforts in the areas of drug development and research on vaccines are essential. Moreover, surveillance programs are key in the validation and modification of the suggested treatment guidelines.
References
World Health Organization (WHO), Department of Reproductive Health and Research (2012) Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. 36 pp
Bolan GA, Sparling PF, Wasserheit JN (2012) The emerging threat of untreatable gonococcal infection. N Eng J Med 366:485–487. doi:10.1056/NEJMp1112456
Centers for Disease Control and Prevention (CDC) (2012) Cephalosporin-resistant Neisseria gonorrhoeae public health response plan. 43 pp
Bodoev IN, Il’ina EN (2015) Molecular mechanisms of drug resistance Neisseria gonorrhoeae: history and prospects. Mol Gen Mikrobiol Virusol 33(3):22–27
Unemo M, del Rio C, Shafer WM (2016) Antimicrobial resistance expressed by Neisseria. gonorrhoeae: a major global public health problem in the 21st century. Microbiol Spectr 4(3). doi:10.1128/microbiolspec.EI10-0009-2015
Fermer C, Kristiansen BE, Sköld O et al (1995) Sulfonamide resistance in Neisseria meningitidis as defined by site-directed mutagenesis could have its origin in other species. J Bacteriol 177(16):4669–4675
Tribuddharat C, Pongpech P, Charoenwatanachokchai A et al (2016) Gonococcal antimicrobial susceptibility and prevalence of bla TEM-1, bla TEM-135 genes in Thailand. Jpn J Infect Dis
Ropp PA, Hu M, Olesky M et al (2002) Mutations in ponA, the gene encoding penicillin-binding protein 1, and a novel locus, penC, are required for high-level chromosomally mediated penicillin resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 46:769–777
de Curraize C, Kumanski S, Micaëlo M et al (2016) Ceftriaxone-resistant Neisseria gonorrhoeae isolates (2010 to 2014) in France characterized by using whole-genome sequencing. Antimicrob Agents Chemother 60(11):6962–6964. doi:10.1128/AAC.01568-16
Serra-Pladevall J, Barberá MJ, Rodriguez S et al (2016) Neisseria gonorrhoeae antimicrobial susceptibility in Barcelona: penA, ponA, mtrR, and porB mutations and NG-MAST sequence types associated with decreased susceptibility to cephalosporins. Eur J Clin Microbiol Infect Dis 35(9):1549–1556. doi:10.1007/s10096-016-2696-7
Morse SA, Johnson SR, Biddle JW et al (1986) High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother 30:664–670
Phillips I (1976) Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet 2:656–657
Davies C, Bussiere DE, Golden BL et al (1998) Ribosomal proteins S5 and L6: high-resolution crystal structures and roles in protein synthesis and antibiotic resistance. J Mol Biol 279:873–888
Ilina EN, Malakhova MV, Bodoev IN et al (2013) Mutation in ribosomal protein S5 leads to spectinomycin resistance in Neisseria gonorrhoeae. Front Microbiol 4:186. doi:10.3389/fmicb.2013.00186
Belland RJ, Morrison SG, Ison C et al (1994) Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol 14:371–380
Kunz AN, Begum AA, Wu H et al (2012) Impact of fluoroquinolone resistance mutations on gonococcal fitness and in vivo selection for compensatory mutations. J Infect Dis 205:1821–1829. doi:10.1093/infdis/jis277
Roberts MC, Chung WO, Roe D et al (1999) Erythromycin-resistant Neisseria gonorrhoeae and oral commensal Neisseria spp. carry known rRNA methylase genes. Antimicrob Agents Chemother 43:1367–1372
Folster JP, Johnson PJ, Jackson L et al (2009) MtrR modulates rpoH expression and levels of antimicrobial resistance in Neisseria gonorrhoeae. J Bacteriol 191:287–297. doi:10.1128/JB.01165-08
Morse SA, Johnson SR, Biddle JW et al (1986) High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother 30:664–670
Unemo M, Shafer WM (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi:10.1128/CMR.00010-14
Ohnishi M, Golparian D, Shimuta K et al (2011) Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi:10.1128/AAC.00325-11
Unemo M, Golparian D, Nicholas R et al (2012) High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi:10.1128/AAC.05760-11
Lahra MM, Ryder N, Whiley DM (2014) A new multidrug-resistant strain of Neisseria gonorrhoeae in Australia. N Eng J Med 371:1850–1851. doi:10.1056/NEJMc1408109
Nakayama S, Shimuta K, Furubayashi K et al (2016) New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother 60(7):4339–4341. doi:10.1128/AAC.00504-16
Martin I, Sawatzky P, Liu G et al (2016) Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerg Infect Dis 22(1):65–67. doi:10.3201/eid2201.151247
Fifer H, Natarajan U, Jones L et al (2016) Failure of dual antimicrobial therapy in treatment of gonorrhea. N Engl J Med 734(35):2504–2506. doi:10.1056/NEJMc1512757
World Health Organization (WHO) (2012) Strategies and laboratory methods for strengthening surveillance of sexually transmitted infections. Available online at: http://apps.who.int/iris/bitstream/10665/75729/1/9789241504478_eng.pdf
Unemo M (2015) Current and future antimicrobial treatment of gonorrhoea—the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect Dis 15:364. doi:10.1186/s12879-015-1029-2
Japanese Society of Sexually Transmitted Infection (2011) Gonococcal infection. Sexually transmitted infections, diagnosis and treatment guidelines 2011. Jpn Sex Transm Dis 22(Suppl 1):52–59
Workowski KA, Bolan GA (2015) Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64:1–137
Public Health Agency of Canada (2013) Canadian guidelines on sexually transmitted infections. Gonococcal infections chapter. Available online at: http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/assets/pdf/section-5-6-eng.pdf
Australasian Sexual Health Alliance (ASHA) (2016) Australian STI management guidelines for use in primary care. Available online at: http://www.sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea#management
Bignell C, Unemo M (2013) 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi:10.1177/0956462412472837
Kirkcaldy RD, Weinstock HS, Moore PC et al (2014) The efficacy and safety of gentamicin plus azithromycin and gemifloxacin plus azithromycin as treatment of uncomplicated gonorrhea. Clin Infect Dis 59:1083–1091. doi:10.1093/cid/ciu521
Biedenbach DJ, Turner LL, Jones RN et al (2012) Activity of JNJ-Q2, a novel fluoroquinolone, tested against Neisseria gonorrhoeae, including ciprofloxacin-resistant strains. Diagn Microbiol Infect Dis 74:204–206. doi:10.1016/j.diagmicrobio.2012.06.006
Roberts MC, Remy JM, Longcor JD et al (2013) In vitro activity of delafloxacin against Neisseria gonorrhoeae clinical isolates. In: Proceedings of the STI & AIDS World Congress, Vienna, Austria, July 2013
Hamasuna R, Yasuda M, Ishikawa K et al (2015) The second nationwide surveillance of the antimicrobial susceptibility of Neisseria gonorrhoeae from male urethritis in Japan, 2012–2013. J Infect Chemother 21:340–345. doi:10.1016/j.jiac.2015.01.010
Kazamori D, Aoi H, Sugimoto K et al (2014) In vitro activity of WQ-3810, a novel fluoroquinolone, against multidrug-resistant and fluoroquinolone-resistant pathogens. Int J Antimicrob Agents 44:443–449. doi:10.1016/j.ijantimicag.2014.07.017
Kerstein K, Fyfe C, Sutcliffe JA et al (2013) Eravacycline (TP-434) is active against susceptible and multidrug-resistant Neisseria gonorrhoeae. In: Proceedings of the 53rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), Denver, Colorado, USA, 10–13 September 2013. Poster E-1181
Zhang YY, Zhou L, Zhu DM et al (2004) In vitro activities of tigecycline against clinical isolates from Shanghai, China. Diagn Microbiol Infect Dis 50:267–281
Fujimoto K, Takemoto K, Hatano K et al (2013) Novel carbapenem antibiotics for parenteral and oral applications: in vitro and in vivo activities of 2-aryl carbapenems and their pharmacokinetics in laboratory animals. Antimicrob Agents Chemother 57:697–707. doi:10.1128/AAC.01051-12
Jacobsson S, Golparian D, Phan LT et al (2015) In vitro activities of the novel bicyclolides modithromycin (EDP-420, EP-013420, S-013420) and EDP-322 against MDR clinical Neisseria gonorrhoeae isolates and international reference strains. J Antimicrob Chemother 70:173–177. doi:10.1093/jac/dku344
Golparian D, Fernandes P, Ohnishi M et al (2012) In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother 56:2739–2742. doi:10.1128/AAC.00036-12
Koeth LM, Difranco-Fisher J (2013) In vitro activity of dalbavancin against Neisseria gonorrhoeae and development of a broth microdilution method. In: Proceedings of IDWeek 2013, San Francisco, California, USA, 2–6 October 2013. Poster 255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of paper, formal consent does not apply.
Informed consent
For this type of paper, informed consent does not apply.
Rights and permissions
About this article
Cite this article
Suay-García, B., Pérez-Gracia, M.T. Drug-resistant Neisseria gonorrhoeae: latest developments. Eur J Clin Microbiol Infect Dis 36, 1065–1071 (2017). https://doi.org/10.1007/s10096-017-2931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2931-x