Abstract
Staphylococcus lugdunensis has emerged as a significant human pathogen, with distinct clinical and microbiological characteristics. Our goal was to identify the virulence factors in S. lugdunensis recovered from infected patients of two Greek hospitals during a six-year period (2008–2013). A collection of 38 S. lugdunensis was tested for biofilm formation, antimicrobial susceptibility, clonal distribution, virulence factors (ica operon, fbl, atlL, vwbl, slush) and antibiotic resistance genes (mecA, ermC) carriage. Strains were classified into pulsotypes by pulsed-field gel electrophoresis (PFGE) of SmaI DNA digests. The majority (22) was isolated from skin and soft tissue infections (SSTIs), nine from deep-sited infections (DSIs), including three bacteraemias and seven from prosthetic device-associated infections (PDAIs). All isolates were oxacillin-susceptible, mecA-negative and fbl-positive. The highest resistance rate was detected for ampicillin (50 %), followed by erythromycin and clindamycin (18.4 %). Fourteen isolates (36.8 %) produced biofilm, whereas 26/38 (68.4 %) carried the ica operon. Biofilm formation was more frequent in isolates from PDAIs. Thirty-six strains (94.7 %) carried atlL and 31 (81.6 %) carried vwbl, whereas slush was detected in 15 (39.5 %). PFGE revealed a low level of genetic diversity: strains were classified into seven pulsotypes, with two major clones (C: 22 and D: nine strains). Type C strains recovered from all infection sites prevailed in biofilm formation and ermC carriage, whereas type D strains associated with SSTIs and DSIs carried more frequently vwbl, slush or both genes. Despite susceptibility to antimicrobials, the clonal expansion and carriage of virulence factors, combined with biofilm-producing ability, render this species an important pathogen that should not be ignored.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coagulase-negative staphylococci (CNS), a part of the normal skin flora, have been recognised as opportunistic pathogens in patients with low immune response or indwelling medical devices. Among them, Staphylococcus lugdunensis has emerged lately as a significant human pathogen with notable clinical and microbiological characteristics that stand out among other CNS. Several infections due to S. lugdunensis have been reported, including native valve endocarditis, skin and soft tissue infections (SSTIs), bloodstream infections, peritonitis, and urinary tract and central nervous system infections [1]. The high level of pathogenicity indicates the presence of various virulence factors.
Despite its aggressive nature, S. lugdunensis, unlike most CNS, has remained remarkably susceptible to a wide range of antimicrobial agents, regardless of the source of infection [2]. In accordance to the exhibited susceptibility, S. lugdunensis has generally been susceptible to oxacillin and polymerase chain reaction (PCR) screening for mecA has frequently yielded negative results. Resistance to beta-lactams due to the production of beta-lactamase has been reported [3], as well as ermC gene carriage, which mediates resistance to macrolides and lincosamides [4, 5].
A variety of microorganisms have the ability to form biofilm, a microbial-derived sessile community characterised by irreversibly attached cells to a substratum, interface or to each other. Bacterial cells are embedded in a matrix of extracellular polymeric substances that they have produced, and exhibit an altered phenotype with respect to growth rate and gene transcription [6]. In staphylococci, biofilm formation is mediated by the production of polysaccharide intercellular adhesin (PIA), encoded by the ica operon or other well-characterised compounds [7, 8]. Biofilm formation is a predominant virulence mechanism employed by S. lugdunensis, whose genome harbours homologues of the ica operon. Biofilm development allows for the deep-sited cells to become more resistant to administered antibiotics and the body’s natural mechanisms, interfering with attempts by the host immune system to clear the infection [1].
The ability of S. lugdunensis to cause endocarditis and prosthetic device-associated infections (PDAIs) suggests that the organism has the potential to interact with host tissues and proteins that may coat foreign surfaces after implantation. A protein has been identified that specifically binds von Willebrand factor (vWf) [9]. vWf is a blood plasma glycoprotein produced by endothelial cells and platelets involved in coagulation, via binding to platelets and subendothelial collagen after vascular injury and stabilising factor VIII of the clotting cascade [10]. The vWf-binding protein of S. lugdunensis (vWbl) is a 2,060-amino-acid protein encoded by the vwbl gene [9]. S. lugdunensis isolates also possess the fbl gene, which encodes a surface-located fibrinogen-binding adhesin, referred as the Fbl protein. It mediates binding to the fibrinogen γ-chain and has close sequence and organisational similarity to clumping factor A of S. aureus [11]. It has been reported that fbl may serve as a species-specific target for S. lugdunensis identification by means of a simple PCR protocol [12].
S. lugdunensis also possess the slush locus, which encodes for haemolytic peptides with delta-toxin-like activity. The S. lugdunensis synergistic haemolysin (SLUSH) is encoded by a locus outside the agr region and comprises three very similar 43-residue peptides with synergistic haemolytic activity with β-toxin [13]. The atlL gene is responsible for the production of an autolysin involved in cell separation, stress-induced autolysis and contributes to bacterial pathogenesis [14].
The aim of the present study was to investigate the biofilm-forming ability, antimicrobial resistance patterns and genetic background of S. lugdunensis recovered from infection sites of patients hospitalised in two hospitals in Greece during a six-year period (2008–2013). Clonal distribution and the frequency of virulence factor-encoding genes were determined.
Materials and methods
Patients and hospitals
A collection of 38 S. lugdunensis isolates recovered from different inpatients hospitalised in a tertiary-care University General Hospital in Patras (UGHP: 37 isolates) and the Pentelis Paediatric Hospital in Athens (PPHA: one isolate), Greece, during a six-year period (2008–2013) were selected to be further analysed. Thirty-six S. lugdunensis were isolated from adults, one from the Neonatal Intensive Care Unit of the UGHP and one from the PPHA that admits patients under the age of 14 years old. The Ethics Committee of the University Hospital of Patras approved this study and waived the need for informed consent (approval no.: 316).
Phenotypic identification and antibiotic susceptibility testing
CNS were identified to the species level using the VITEK 2 Advanced Expert System (bioMérieux, Marcy l’Etoile, France). Susceptibility to cefoxitin (FOX), ampicillin (AMP), rifampicin (RIF), erythromycin (E), clindamycin (CC), kanamycin (KAN), tetracycline (TE), gentamicin (GM), ciprofloxacin (CIP), fusidic acid (FA) and sulphamethoxazole/trimethoprim (SXT) was tested by the disc diffusion method according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines [15]. Macrolide–lincosamide–streptogramin B (MLSB) resistance phenotype was investigated by the combined erythromycin and clindamycin disc method (D-test) [15]. The minimum inhibitory concentrations (MICs) of oxacillin (OX), vancomycin (VA), teicoplanin (TEC), linezolid (LNZ) and daptomycin (DAP) were determined by the Etest (bioMérieux). Isolates exhibiting a resistance phenotype to at least three different classes of antimicrobials were considered multidrug-resistant. Beta-lactamase production was tested by the nitrocefin assay (Becton Dickinson, Franklin Lakes, NJ, USA). Biofilm formation was tested by the quantitative microtitre plate assay using the reference S. epidermidis ATCC35984 (RP62A), slime producing/ica-positive strain, and ATCC12228, slime-negative/ica-negative strain, as positive and negative controls, respectively [16].
Molecular analysis
Amplification of the gene encoding the fibronectin-binding protein (fbl) was performed for the verification of species identification [12]. The presence of mecA [17], one gene of the ica operon (icaA) [18], the synergistic haemolysin (slush) [19], the von-Willebrand binding factor (vwbl) [19] and the ribosomal methylase gene ermC [5], encoding resistance to erythromycin, was investigated by PCRs with specific primers.
For PCR-based detection of the gene encoding the major cell wall autolysin (atlL), a pair of gene-specific primers was synthesised. The primers were as follows: 5′-CCATCAACACCTACAAATCC-3′ as the forward primer and 5′-CAGCCAGATTTACCATTCAC-3′ as the reverse primer, amplifying a 449-bp region. Thermal cycling conditions included an initial denaturation step (10 min at 94 °C), followed by 35 cycles of amplification (denaturation for 30 s at 95 °C, annealing for 30 s at 56 °C and extension for 30 s at 72 °C). The reaction was terminated with a 10-min final extension step at 72 °C. PCR products were analysed by electrophoresis into 1 % agarose gels.
Clonal identification
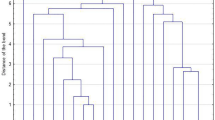
The strains were classified into pulsotypes by pulsed-field gel electrophoresis (PFGE) of chromosomal DNA SmaI digests [20], named by capital letters. A dendrogram comparing the molecular weights of DNA fragments was performed by FPQuest software version 4.5 (Bio-Rad Laboratories, Inc.). According to criteria established by Miragaia et al., patterns differing by less than 79 % (corresponding to a difference of less than seven bands) were considered to belong to the same PFGE type [21].
Results
A collection of 38 S. lugdunensis clinical isolates was studied. The majority of strains (22/38, 57.9 %) derived from SSTIs (wounds, abscesses and skin infections), nine strains (23.7 %) from deep-sited infections (DSIs, including three bacteraemias, osteomyelitis tissue samples, synovial and peritoneal fluids) and seven strains (18.4 %) from PDAIs (intravenous and peritoneal catheters) (Table 1). Two S. lugdunensis strains were recovered from children.
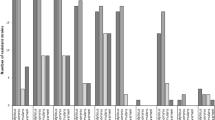
All 38 isolates were fbl-positive, cefoxitin- and oxacillin-susceptible (MICs ≤1.5 μg/mL), and did not carry the mecA gene. Moreover, all isolates were susceptible to linezolid (MICs ≤1 μg/mL), daptomycin (MICs ≤0.75 μg/mL), vancomycin (MICs ≤2 μg/mL), teicoplanin (MICs ≤2 μg/mL), gentamicin, kanamycin, ciprofloxacin and rifampicin. The higher resistance rates were detected for ampicillin (19/38, 50 %), erythromycin (7/38, 18.4 %) and clindamycin (7/38, 18.4 %). Only four strains (10.5 %) were multi-resistant, whereas all of them belonged to the same PFGE type (C). Nineteen isolates produced beta-lactamase (Table 2). Seven erythromycin- and clindamycin-resistant strains expressing constitutive resistance carried the ermC gene.
In total, 14/38 isolates (36.8 %) produced biofilm, whereas 26/38 (68.4 %) carried the ica operon. Among biofilm-producers, ica operon carriage was detected in 10/14 isolates (71.4 %). Of the 24 remaining biofilm-negative S. lugdunensis, 16 (66.7 %) were also found to carry the ica operon. The four biofilm-positive ica-deficient isolates belonged to the same PFGE type, C, and carried the atlL gene. Biofilm formation and atlL gene carriage were more frequent in isolates from PDAIs, whereas vwbl and slush genes were more frequent in isolates from DSIs (Table 1).
PFGE typing revealed a low level of genetic diversity; the 38 S. lugdunensis strains were classified into seven pulsotypes. Thirty-one isolates (81.6 %) were classified into two major clones, C and D, consisting of 22 and nine strains, respectively. A dendrogram showing the main S. lugdunensis pulsotypes is presented in Fig. 1. All S. lugdunensis recovered from bacteraemias (three strains) belonged to the main PFGE type, C. Pulsotype D strains were recovered from SSTIs and DSIs (Table 1). Strains belonging to clone C prevailed in biofilm formation and in ermC carriage as compared to pulsotype D (Table 2).
Among the virulence genes detected, fbl and atlL predominated in this study. All S. lugdunensis carried fbl, as expected, whereas atlL was identified in 36/38 isolates (94.7 %, Table 1). Thirty-one (81.6 %) S. lugdunensis carried vwbl, while slush was detected in only 15 strains (39.5 %, Table 1). Type D strains carried more frequently vwbl (88.9 %) and slush (77.8 %, Table 2). Six out of nine type D and four out of 22 type C strains carried both vwbl and slush genes. Five out of six type D strains carrying both genes were recovered from SSTIs, whereas type C strains were isolated from variable infection sites. PFGE type C was identified in both hospitals (UGHP and PPHA), located in different areas of the country.
Discussion
S. lugdunensis is found as a skin commensal in healthy individuals, but has also been implicated in invasive diseases, especially native and prosthetic cardiac valve endocarditis, meningitis, peritonitis and SSTIs [1]. In our study, 38 S. lugdunensis were isolated during a six-year period in two Greek hospitals. Only three patients had bacteraemia, whereas skin and soft tissue were the main infection sites.
Various studies have demonstrated increased resistance of CNS to antimicrobials [22]. On the contrary, S. lugdunensis remains remarkably susceptible to most antistaphylococcal agents. An analysis of 28 S. lugdunensis from patients with bacteraemia in Switzerland concluded that most strains (82 %) were penicillin-susceptible and none was oxacillin- or multi-resistant [23]. Likewise, all isolates in our collection were susceptible to cefoxitin and oxacillin (methicillin-susceptible S. lugdunensis). Moreover, no isolate resistant to gentamicin, kanamycin, ciprofloxacin, rifampicin, vancomycin, teicoplanin, linezolid and daptomycin was found. Despite the high level of susceptibility, 50 % of the studied population was resistant to ampicillin, whereas four type C strains were multi-resistant without correlation to any outbreak; three of them were isolated from skin infections in 2010 and one from an intravenous catheter in 2013. Two multi-resistant strains were recovered from patients hospitalised in the Nephrology Department, one from the Vascular Surgery Clinic and one from the Outpatients Department (UGHP). In contrast to other CNS, S. lugdunensis produces beta-lactamase in a relatively low rate (19/38, 50 %), confirming the low level of resistance. The carriage of ermC was also not prevalent (7/38, 18.4 %), confirming the high level of susceptibility to macrolides.
A major factor in the pathogenesis of staphylococcal infections is biofilm formation [6]. As compared to the planktonic phase, biofilm confers to staphylococci increased mechanical, metabolic, immune and antibiotic resistance. Soon after the description of S. lugdunensis, Lambe et al. showed that a glycocalyx, described as a negatively charged polysaccharide matrix surrounding bacterial microcolonies, could be visualised by transmission electron microscopy when cells were stained with ruthenium red, a stain with affinity for polyanionic structures [24]. In the present study, 14 out of 38 isolates (36.8 %) produced biofilm. S. lugdunensis isolated from PDAIs formed biofilm in a higher rate as compared to other infection sites (Table 1), confirming that the presence of a foreign surface is important for the initial bacterial attachment, which is the first step in biofilm formation and the pathogenesis of the infection. A locus with homology to the S. aureus and S. epidermidis ica operon has been identified in S. lugdunensis, but no direct relation between ica carriage and biofilm formation was determined in our collection. It has been reported that S. lugdunensis forms biofilm, but its biofilm extracellular matrix is predominantly proteinaceous and independent of poly-N-acetylglucosamine production, which is encoded by ica [25]. As published by Pereira et al., investigating biofilm production in 23 S. lugdunensis clinical isolates, even though all of them carried ica, only 14 strains were biofilm-positive and in a detachment assay, using proteolytic enzymes to analyse biofilm composition, they showed protein-mediated biofilm structure [26]. Similarly, proteinaceous biofilm composition was identified in the present study, in which among the 26 ica-positive strains, only ten produced biofilm, whereas the remaining four biofilm-producers carried atlL.
PFGE analysis of S. lugdunensis strains revealed that, although they were recovered from different patients and treated in different wards and hospitals, two main clones (C and D) predominated during this six-year period. The three bacteraemic as well as the four multi-resistant isolates belonged to PFGE type C. In a study published by Hellbacher et al., 39 S. lugdunensis were classified into nine pulsotypes, and the majority (56 %) belonged to one main PFGE type [27]. In our collection, the main pulsotype, C, also comprised the majority of strains (22/38, 57.9 %), indicating a low degree of genetic diversity. This may indicate that the S. lugdunensis genome is highly conserved or that specific clones are more likely to cause invasive infections. Clone C predominated, regardless of the site of infection. The nine isolates that comprised pulsotype D (23.7 %) originated from SSTIs and DSIs, but not PDAIs.
As reported by Chatzigeorgiou et al., fbl may serve as a species-specific target for S. lugdunensis identification by means of a simple PCR protocol [12]. In our study, the VITEK 2 Advanced Expert System (bioMérieux) used to phenotypically identify CNS species correctly detected all S. lugdunensis proven by the fbl PCR assay.
An important virulence factor in our collection was found to be atlL, which was detected in the majority (94.7 %) of isolates, causing mainly PDAIs and SSTIs, including all four biofilm-positive/ica-deficient S. lugdunensis. AtlL is a major autolysin involved in S. lugdunensis cell division. Quantitative PCR of atlL gene expression indicates that it is transcribed throughout all phases of growth (early exponential, mid-exponential and stationary growth phases), with an increased level (5.5-fold) at the early exponential stage, which is consistent with the hypothesis of an involvement in cell division [28]. Gibert et al., investigating the role of AtlL by an atlL-inactivated S. lugdunensis mutant, concluded that this protein could act as an autolysin/adhesin, conferring initial bacterial attachment and release of extracellular DNA, being, thus, a major component in PIA-independent proteinaceous S. lugdunensis biofilms [14].
The majority of S. lugdunensis studied (81.6 %) also carried vwbl, which encodes the putative cell surface protein, vWbl. The purified protein has an overall organisation typical of staphylococcal cell surface proteins, with an N-terminal signal peptide and a C-terminal cell wall sorting signal. A high prevalence of vwbl was also reported by Nilsson et al., where Southern blot analysis showed that vwbl was present in all 12 S. lugdunensis strains tested [9].
Several staphylococcal species express a synergistic activity, which potentiates haemolysis by β-haemolysin. Haemolysis of red blood cells is an important pathogenicity factor. In S. lugdunensis, this activity results from the production of three small peptides coded by the slush locus [13]. In our study collection, slush was present in 15 isolates (39.5 %), whereas the analysis of 58 S. lugdunensis clinical isolates by Szabados et al., detected it in 29 isolates (50 %) [19]. The presence of other genes encoding haemolysins should also be considered.
In conclusion, S. lugdunensis stands out between other CNS and has been fairly well described as ‘a wolf in sheep’s clothing’ [1]. Despite its susceptibility to antimicrobials, clonal expansion, carriage of virulence factors such as fbl, ica, atlL, vwbl and slush, combined with biofilm-producing ability, render this species an important pathogen that should not be ignored in clinical practice.
References
Frank KL, Del Pozo JL, Patel R (2008) From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev 21(1):111–133. doi:10.1128/CMR.00036-07
Frank KL, Reichert EJ, Piper KE, Patel R (2007) In vitro effects of antimicrobial agents on planktonic and biofilm forms of Staphylococcus lugdunensis clinical isolates. Antimicrob Agents Chemother 51(3):888–895. doi:10.1128/AAC.01052-06
van der Mee-Marquet N, Achard A, Mereghetti L, Danton A, Minier M, Quentin R (2003) Staphylococcus lugdunensis infections: high frequency of inguinal area carriage. J Clin Microbiol 41(4):1404–1409
Liu C, Shen D, Guo J, Wang K, Wang H, Yan Z, Chen R, Ye L (2012) Clinical and microbiological characterization of Staphylococcus lugdunensis isolates obtained from clinical specimens in a hospital in China. BMC Microbiol 12:168. doi:10.1186/1471-2180-12-168
Spiliopoulou I, Petinaki E, Papandreou P, Dimitracopoulos G (2004) erm(C) is the predominant genetic determinant for the expression of resistance to macrolides among methicillin-resistant Staphylococcus aureus clinical isolates in Greece. J Antimicrob Chemother 53(5):814–817. doi:10.1093/jac/dkh197
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R (1996) The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178(1):175–183
Spiliopoulou AI, Krevvata MI, Kolonitsiou F, Harris LG, Wilkinson TS, Davies AP, Dimitracopoulos GO, Karamanos NK, Mack D, Anastassiou ED (2012) An extracellular Staphylococcus epidermidis polysaccharide: relation to Polysaccharide Intercellular Adhesin and its implication in phagocytosis. BMC Microbiol 12:76. doi:10.1186/1471-2180-12-76
Nilsson M, Bjerketorp J, Wiebensjö A, Ljungh A, Frykberg L, Guss B (2004) A von Willebrand factor-binding protein from Staphylococcus lugdunensis. FEMS Microbiol Lett 234(1):155–161. doi:10.1016/j.femsle.2004.03.024
Vischer UM, de Moerloose P (1999) von Willebrand factor: from cell biology to the clinical management of von Willebrand’s disease. Crit Rev Oncol Hematol 30(2):93–109
Mitchell J, Tristan A, Foster TJ (2004) Characterization of the fibrinogen-binding surface protein Fbl of Staphylococcus lugdunensis. Microbiology 150(Pt 11):3831–3841. doi:10.1099/mic.0.27337-0
Chatzigeorgiou KS, Siafakas N, Petinaki E, Zerva L (2010) fbl gene as a species-specific target for Staphylococcus lugdunensis identification. J Clin Lab Anal 24(2):119–122. doi:10.1002/jcla.20352
Donvito B, Etienne J, Greenland T, Mouren C, Delorme V, Vandenesch F (1997) Distribution of the synergistic haemolysin genes hld and slush with respect to agr in human staphylococci. FEMS Microbiol Lett 151(2):139–144
Gibert L, Didi J, Marlinghaus L, Lesouhaitier O, Legris S, Szabados F, Pons JL, Pestel-Caron M (2014) The major autolysin of Staphylococcus lugdunensis, AtlL, is involved in cell separation, stress-induced autolysis and contributes to bacterial pathogenesis. FEMS Microbiol Lett 352(1):78–86. doi:10.1111/1574-6968.12374
The European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0, 2014. http://www.eucast.org
Stepanović S, Vuković D, Hola V, Di Bonaventura G, Djukić S, Cirković I, Ruzicka F (2007) Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 115(8):891–899. doi:10.1111/j.1600-0463.2007.apm_630.x
Murakami K, Minamide W, Wada K, Nakamura E, Teraoka H, Watanabe S (1991) Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 29(10):2240–2244
Sandoe JA, Longshaw CM (2001) Ventriculoperitoneal shunt infection caused by Staphylococcus lugdunensis. Clin Microbiol Infect 7(7):385–387
Szabados F, Nowotny Y, Marlinghaus L, Korte M, Neumann S, Kaase M, Gatermann SG (2011) Occurrence of genes of putative fibrinogen binding proteins and hemolysins, as well as of their phenotypic correlates in isolates of S. lugdunensis of different origins. BMC Res Notes 4:113. doi:10.1186/1756-0500-4-113
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33(9):2233–2239
Miragaia M, Carriço JA, Thomas JC, Couto I, Enright MC, de Lencastre H (2008) Comparison of molecular typing methods for characterization of Staphylococcus epidermidis: proposal for clone definition. J Clin Microbiol 46(1):118–129. doi:10.1128/JCM.01685-07
Santos Sanches I, Mato R, de Lencastre H, Tomasz A; CEM/NET Collaborators and the International Collaborators (2000) Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb Drug Resist 6(3):199–211
Zinkernagel AS, Zinkernagel MS, Elzi MV, Genoni M, Gubler J, Zbinden R, Mueller NJ (2008) Significance of Staphylococcus lugdunensis bacteremia: report of 28 cases and review of the literature. Infection 36(4):314–321. doi:10.1007/s15010-008-7287-9
Lambe DW Jr, Jeffery C, Ferguson KP, Cooper MD (1994) Examination of the glycocalyx of four species of Staphylococcus by transmission electron microscopy and image analysis. Microbios 78(316):133–143
Frank KL, Patel R (2007) Poly-N-acetylglucosamine is not a major component of the extracellular matrix in biofilms formed by icaADBC-positive Staphylococcus lugdunensis isolates. Infect Immun 75(10):4728–4742. doi:10.1128/IAI.00640-07
Pereira EM, Teixeira CA, Alvarenga AL, Schuenck RP, Giambiagi-Demarval M, Holandino C, Mattos-Guaraldi AL, dos Santos KR (2012) A Brazilian lineage of Staphylococcus lugdunensis presenting rough colony morphology may adhere to and invade lung epithelial cells. J Med Microbiol 61(Pt 4):463–469. doi:10.1099/jmm.0.033001-0
Hellbacher C, Törnqvist E, Söderquist B (2006) Staphylococcus lugdunensis: clinical spectrum, antibiotic susceptibility, and phenotypic and genotypic patterns of 39 isolates. Clin Microbiol Infect 12(1):43–49. doi:10.1111/j.1469-0691.2005.01296.x
Bourgeois I, Camiade E, Biswas R, Courtin P, Gibert L, Götz F, Chapot-Chartier MP, Pons JL, Pestel-Caron M (2009) Characterization of AtlL, a bifunctional autolysin of Staphylococcus lugdunensis with N-acetylglucosaminidase and N-acetylmuramoyl-l-alanine amidase activities. FEMS Microbiol Lett 290(1):105–113. doi:10.1111/j.1574-6968.2008.01414.x
Acknowledgements
We thank Anastasia Spiliopoulou MD, PhD for her assistance in collecting the isolates. The Ethics Committee of the University Hospital of Patras approved this study and waived the need for informed consent (approval no.: 316). Part of this work was presented as a poster presentation at the 24th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID 2014), May 2014, Barcelona, Spain.
Funding
This research was supported by funding from the National Staphylococcal Reference Laboratory, Greece, under the scientific responsibility of I.S. and E.D.A. (grant C954, Hellenic Centre for Disease Control and Prevention, HCDCP/KEELPNO).
Conflict of interest
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giormezis, N., Kolonitsiou, F., Makri, A. et al. Virulence factors among Staphylococcus lugdunensis are associated with infection sites and clonal spread. Eur J Clin Microbiol Infect Dis 34, 773–778 (2015). https://doi.org/10.1007/s10096-014-2291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2291-8