Abstract
We aimed to validate a severity grading score (SGS) system for predicting the course of disease and fatality in Crimean-Congo hemorrhagic fever (CCHF). This SGS was established using several variables that were assumed to be associated with mortality and had clinical importance. We included patients diagnosed with CCHF from different centers. Patients who had symptoms of CCHF for <5 days were included. The patients were grouped into three categories according to mortality risk. An SGS ≤4 showed no association with mortality [n = 323 (79.9 % of the total study population), and all survived]. An SGS between 5 and 8 points was classified into the intermediate risk group (20 %), and 14 of 70 patients in this group died. An SGS ≥9 was classified as the high risk of mortality group and 11 of 11 patients in this group died (p = 0.001). The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for an SGS >9 points at admission were 96, 100, 97, 100, and 44 %, respectively. This SGS system may help appropriate the triage of patients, decrease the cost of treatment, and improve the functionality of healthcare staff. The present study is the first investigation about the validation of an SGS system in patients with CCHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crimean-Congo hemorrhagic fever (CCHF) is an acute viral hemorrhagic disease with a mortality rate between 3 and 30 %. The virus that causes CCHF belongs to the Nairovirus genus of the Bunyaviridae family. CCHF was first described in the 1940s in the Crimean peninsula of the former Soviet Union; however, it is now endemic in approximately 30 countries in Africa, Asia, Europe, and the Middle East [1]. Up to 2009, 4,448 confirmed CCHF cases have been reported in Turkey [2].
The clinical course and outcome of CCHF infection differ considerably among cases [3]. Although ribavirin has been recommended for the prophylaxis and therapy of CCHF, its efficacy remains debatable. Most CCHF patients receive only supportive therapy, and these patients must be monitored closely for effective support [4, 5]. Several studies have evaluated the effect of several clinical and laboratory findings on the mortality of CCHF. However, physicians in primary or secondary care hospitals face difficulties in predicting the disease course and determining which patients need general supportive therapy, including replacement of basic blood products and balance of fluids and electrolytes, and which patients need more advanced supportive therapy that can be provided at a tertiary care hospital, such as thrombocyte apheresis and intensive care monitoring. In a previous study, Bakir et al. evaluated patients on the first day of hospitalization, and they composed a severity grading score (SGS) according to the clinical and laboratory findings, commented on the course of the disease, and predicted mortality in patients with CCHF using the SGS system [6] . They suggested that this scoring system might not only help decide which patients should be referred to a tertiary care hospital, but also decrease the cost of therapy and improve the functionality of healthcare staff.
In the previous study, an SGS system was created using the clinical and laboratory findings of CCHF patients from a single center [6]. In this study, we aimed to validate the SGS system using data from multiple centers that are responsible for the follow-up and treatment of a significant proportion of the CCHF patients in Turkey. Furthermore, we aimed to use the clinical and laboratory findings to determine which patients could be followed up in a secondary care hospital and which patients should be referred to a tertiary care hospital for monitoring in the intensive care unit or for platelet apheresis.
Materials and methods
Study design and patients
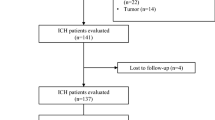
This prospective study was conducted in six different centers, including the Cumhuriyet University Hospital, Karadeniz Technical University Hospital, 19 Mayıs University Hospital, Gaziosmanpasa University Hospital, Ankara Numune Training and Research Hospital, and Tokat State Hospital, between April 1, 2012 and September 30, 2012. These hospitals are located in a highly endemic region for CCHF, and they follow-up on and treat a significant portion of CCHF patients in Turkey. Patients with suspected CCHF according to clinical and laboratory findings that were referred to these hospitals between April 1, 2012 and September 30, 2012 were included. All of the referred patients were hospitalized at the clinical ward of the departments of infectious disease and clinical microbiology, and followed up in these centers until definitive diagnosis. Of note, patients who had symptoms for ≤5 days were included and patients who had symptoms for ≥6 days were excluded, since the current study aimed to predict the course of the disease in the early period. Hence, 404 confirmed CCHF patients were considered for the analysis. All patients diagnosed with CCHF were followed up until death or complete recovery. The study protocol was approved by the Human Ethics Committee of the Cumhuriyet University School of Medicine.
Diagnosis of CCHF
Acute-phase serum samples were sent to the Virology Laboratory of Refik Saydam Hygiene Center in Ankara, Turkey, for serological and virological analyses. All serum specimens were stored at −70 °C until testing. Definitive diagnosis of CCHF infection was based on typical clinical and epidemiological findings: detection of CCHF virus-specific IgM by enzyme-linked immunosorbent assay (ELISA) and/or by detection of genomic segments of the CCHF virus by reverse transcription-polymerase chain reaction (RT-PCR) in the acute phase of the disease.
SGS system
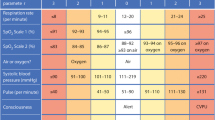
The SGS system was established using variables that were published in a previous study, with minor differences [6]. In the previous study, we used the disseminated intravascular coagulation (DIC) score for the SGS. In this study, we did not evaluate the DIC score because not all centers collect all of the parameters, such as fibrinogen and D-dimers, that are necessary for this evaluation. Instead, we evaluated parameters such as activated partial thromboplastin time (aPTT) and international normalized ratio (INR). The variables used in the SGS system and the points for each variable are listed in Table 1. The maximum SGS obtained in this study was 14 points. According to the SGS points at hospital admission, the patients were classified into three risk groups for mortality: low (0–4 points), intermediate (5–8 points), and high (9–14 points).
Statistical analysis
The Statistical Package for the Social Sciences (SPSS) version 14 for Windows (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Non-parametric data were expressed as median (min–max) and categorical data as percentages. The SGS for each patient was analyzed using the Mann–Whitney U-test in patients with fatal and non-fatal CCHF infection. Proportions for categorical variables were compared using the Chi-square test. The accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the SGS were calculated. A p-value <0.05 was considered significant in all analyses.
Results
In this study, we evaluated the clinical and laboratory findings of 404 patients from six different centers. Of the patients, 237 (58.7 %) were males and 167 (41.3 %) were females, with a median age of 47 years (range 16–90 years), and a median follow-up time of 7 days (range 1–30 days). In addition, 25 patients (6.2 %) died, and 379 were discharged. Only the RT-PCR results were positive for 201 patients (49.8 %), only the CCHF virus-specific IgM was positive for 70 patients (17.3 %), and both RT-PCR and CCHF virus-specific IgM were positive for 133 patients (32.9 %). Table 2 shows the prognostic effects of each parameter in the SGS system. The median SGS at hospital admission for all patients was 2 (range 0–12), and it was higher for the patients who died during the follow-up period: 8 (range 5–12). On the other hand, the score for patients who survived was 2 (range 0–8) (p < 0.001). SGS ≤ 4 was not associated with mortality [n = 323 (79.9 % of the total study population), and all survived]. An SGS between 5 and 8 points was classified as intermediate risk of mortality (20 %), and 14 of the 70 patients in this group died. SGS ≥ 9 was associated with high risk of mortality (100 %), and all of the 11 patients in this group died (p = 0.001) (Table 3). Approximately 50 % (12 of 25 patients) of the deaths occurred within 72 h of admission.
The sensitivity, specificity, accuracy, PPV, and NPV for an SGS > 9 points at admission were 96, 100, 97, 100, and 44 %, respectively.
Discussion
The typical course of CCHF infection has been classified into four distinct phases: incubation, prehemorrhagic, hemorrhagic, and convalescence [7]. However, patients with CCHF may present with a clinical profile spectrum varying from mild, which includes flu-like symptoms and no hemorrhagic manifestations, to severe, which includes flu-like symptoms and hemorrhagic manifestations. Hemorrhagic findings develop within 3–6 days of disease onset in severe cases [7, 8]. Several studies have reported prognostic factors for CCHF from various parts of the world. Swanepoel et al. reported that the fatality risk will be 90 % if one of the following criteria are fulfilled: leukocyte count >10,000/mm3, platelet count <20,000/mm3, aspartate aminotransferase (AST) level >200 U/L, alanine transaminase (ALT) level >150 U/L, aPPT > 60 s, and fibrinogen level <110 mg/dL during the first 5 days of illness [9]. Cevik et al. [8] emphasized that thrombocytopenia of <20 × 109/L [hazard ratio (HR) 9.67], a prolonged aPTT > 60 s (HR 11.62), the existence of melena (HR 6.39), and somnolence (HR 6.30) were independently associated with mortality. Yilmaz et al. [10] reported that the risk of a severe clinical course in CCHF patients increased 2.59 and 3.93 times, respectively, in the presence of platelet counts and hemoglobin levels below cut-off values. Moreover, the risk increased to 2.95, 2.92, and 3.47 times when the levels of INR, AST, and C-reactive protein, respectively, were above the predetermined cut-off values. Tasdelen Fisgin et al. [11] reported advanced age as an early indicator of poor prognosis in patients with CCHF. According to these studies, the mean ALT, AST, lactate dehydrogenase, creatine phosphokinase, INR, and white blood cell levels were higher; the mean platelet was lower; and the mean prothrombin time and aPTT were longer in the fatal cases than in the non-fatal cases [3, 6, 8, 11–15]. In addition, petechia/ecchymosis, bleeding from multiple sites, and somnolence were reported to be more common in the fatal cases than in the non-fatal cases [8, 14, 15]. Predicting the clinical course of CCHF disease may be of lifesaving importance because it enables accurate and appropriate management of the supportive treatment. However, it is difficult to comment on the severity of the disease and to determine which patients should be referred to a tertiary care hospital. For this purpose, Bakir et al. composed an SGS system to predict the course of the disease and fatality in CCHF patients for the first time [6]. They used mortality-associated risk factors described in the literature and clinical observations to determine the parameters within the SGS system. The patients were grouped into three categories according to the mortality risk as follows: low or no risk, intermediate risk, and high risk. They demonstrated that SGS < 5 showed no association with mortality (n = 158 cases, and all patients survived), and this group constituted 66.7 % of the patients with CCHF. They showed that an SGS of 6–10 showed a moderate risk of mortality (10 %), and 7 of 70 patients in this group died. Furthermore, an SGS >11 was shown to indicate a high risk of mortality (67 %), and 6 of 9 patients in this group died (p = 0.001). The sensitivity, specificity, accuracy, PPV, and NPV for an SGS > 11 points were 67, 100, 98, 100, and 98 %, respectively [6].
After our study entitled “A new perspective to determine the severity of cases with Crimean-Congo hemorrhagic fever” in 2012, a new study to determine the severity of cases with CCHF was presented by Dokuzoguz et al. [16] in 2013. The authors showed that the severity scoring index (SSI) predicts mortality effectively in their study. For calculation of the SSI, five parameters were considered, as follows: platelet count, aPPT, fibrinogen, bleeding, and somnolence. Then, the scores of the patients were calculated by the algebraic sum of the points, defined for the parameters. They classified patients into three groups as mild, moderate, and severe cases. The cut-off point between moderate and severe cases was set at 10, since the case fatality rate was >50 % in the case of SSI > 10. The results of the current study seem to support this previous observation. To the best of our knowledge, our study was the first investigation on the validation of the SSI in patients with CCHF.
In our previous study, the CCHF patients were classified into three groups according to the risk of mortality. The first group, which did not show any mortality, comprised 66.7 % of the patients with CCHF. These patients could be monitored in a secondary care hospital. However, our previous study included patients from only one center. In the present study, we evaluated and validated the SGS using 404 patients from six different centers located in a highly endemic region. According to our new SGS scale, the low or no risk group included 79.9 % of all cases, which was higher than the proportion (66.7 %) observed in our previous study. Our previous study included patients from only one center, and that hospital serves as a tertiary care hospital; this study included patients from six different centers, including both secondary and tertiary care hospitals. In our previous study, all patients were followed up in a tertiary care hospital, and a significant portion of those patients was referred from the secondary care hospital because the patients required advanced supportive therapy. In this study, the patients were recruited from both secondary and tertiary care hospitals. Therefore, a significant portion of these patients had a mild disease course; this might have caused the differences in the proportion of patients in the first (low or no risk) group. We did not evaluate the DIC score because fibrinogen and D-dimer levels that are necessary to determine the DIC score were not adequately evaluated in all of the centers. Therefore, we considered parameters such as aPTT and INR. Hence, this study utilized aPTT and INR instead of the DIC score.
There are some limitations of our study worthwhile mentioning. First of all, we did not check for the viral load of CCHF patients, though high viral load was shown to be associated with excess mortality in some previous studies [17, 18]. Of note, the determination of viral load was not practical in the field, and we considered that it was not appropriate for such a scoring system. Secondly, there may be differences in supportive therapy administered to the patients, including blood transfusion, and blood product and fluid replacement, due to the multicenter design of the study, which left therapy to the discretion of the primary physician. However, all participating centers have served the best of medical care to their CCHF patients since the beginning of the epidemic in Turkey, and, hence, all could possibly be considered as experienced centers.
In conclusion, risk stratification of patients according to the SGS is important in terms of patient management. Because of disease severity and mortality risk, the practitioners of primary and secondary care facilities refer patients with CCHF to tertiary care hospitals, and superfluous referral of patients to tertiary care hospitals might be prevented by an SGS-based triage. If patients with moderate and high SGS can be identified, they can then be referred to tertiary care hospitals, which have intensive care units and blood centers that can perform platelet apheresis. Subsequently, the percentage of patients who are referred to tertiary care hospitals will decrease. This approach might also decrease care costs and improve the functionality of the healthcare staff. In addition, patients with a high risk of death could be identified early. Furthermore, we believe that the SGS might be useful in the therapeutic decision-making process; hence, the recommended treatment options (ribavirin, steroid, plasma exchange, etc.) might be administered as early as possible.
References
Ergönül O (2006) Crimean-Congo haemorrhagic fever. Lancet Infect Dis 6:203–214
The Turkish Medical Association Publications (Türk Tabipleri Birligi Yayinlari) (2011) Scientific assessment report of the Crimean-Congo haemorrhagic fever. Available online at: http://www.ttb.org.tr/kutuphane/kirim_kongo_rpr.pdf. Accessed 17 July 2014
Ergonul O, Celikbas A, Baykam N, Eren S, Dokuzoguz B (2006) Analysis of risk-factors among patients with Crimean-Congo haemorrhagic fever virus infection: severity criteria revisited. Clin Microbiol Infect 12:551–554
Koksal I, Yilmaz G, Aksoy F, Aydin H, Yavuz I, Iskender S, Akcay K, Erensoy S, Caylan R, Aydin K (2010) The efficacy of ribavirin in the treatment of Crimean-Congo hemorrhagic fever in Eastern Black Sea region in Turkey. J Clin Virol 47:65–68
Keshtkar-Jahromi M, Kuhn JH, Christova I, Bradfute SB, Jahrling PB, Bavari S (2011) Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antiviral Res 90:85–92
Bakir M, Engin A, Gozel MG, Elaldi N, Kilickap S, Cinar Z (2012) A new perspective to determine the severity of cases with Crimean-Congo hemorrhagic fever. J Vector Borne Dis 49:105–110
Whitehouse CA (2004) Crimean-Congo hemorrhagic fever. Antiviral Res 64:145–160
Cevik MA, Erbay A, Bodur H, Gülderen E, Baştuğ A, Kubar A, Akinci E (2008) Clinical and laboratory features of Crimean-Congo hemorrhagic fever: predictors of fatality. Int J Infect Dis 12:374–379
Swanepoel R, Gill DE, Shepherd AJ, Leman PA, Mynhardt JH, Harvey S (1989) The clinical pathology of Crimean-Congo hemorrhagic fever. Rev Infect Dis 11:S794–S800
Yilmaz G, Koksal I, Topbas M, Yilmaz H, Aksoy F (2010) The effectiveness of routine laboratory findings in determining disease severity in patients with Crimean-Congo hemorrhagic fever: severity prediction criteria. J Clin Virol 47:361–365
Tasdelen Fisgin N, Tanyel E, Doganci L, Tulek N (2009) Risk factors for fatality in patients with Crimean-Congo haemorrhagic fever. Trop Doct 39:158–160
Bakir M, Ugurlu M, Dokuzoguz B, Bodur H, Tasyaran MA, Vahaboglu H; Turkish CCHF Study Group (2005) Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: a multicentre study of clinical features and outcome measures. J Med Microbiol 54:385–389
Onguru P, Dagdas S, Bodur H, Yilmaz M, Akinci E, Eren S, Özet G (2010) Coagulopathy parameters in patients with Crimean-Congo hemorrhagic fever and its relation with mortality. J Clin Lab Anal 24:163–166
Hatipoglu CA, Bulut C, Yetkin MA, Ertem GT, Erdinc FS, Kilic EK, Sari T, Kinikli S, Oral B, Demiroz AP (2010) Evaluation of clinical and laboratory predictors of fatality in patients with Crimean-Congo haemorrhagic fever in a tertiary care hospital in Turkey. Scand J Infect Dis 42:516–521
Ozkurt Z, Kiki I, Erol S, Erdem F, Yilmaz N, Parlak M, Gundogdu M, Tasyaran MA (2006) Crimean-Congo hemorrhagic fever in Eastern Turkey: clinical features, risk factors and efficacy of ribavirin therapy. J Infect 52:207–215
Dokuzoguz B, Celikbas AK, Gök ŞE, Baykam N, Eroglu MN, Ergönül Ö (2013) Severity scoring index for Crimean-Congo hemorrhagic fever and the impact of ribavirin and corticosteroids on fatality. Clin Infect Dis 57:1270–1274
Cevik MA, Erbay A, Bodur H, Eren SS, Akinci E, Sener K, Ongürü P, Kubar A (2007) Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin Infect Dis 45(7):e96–e100
Wölfel R, Paweska JT, Petersen N, Grobbelaar AA, Leman PA, Hewson R, Georges-Courbot MC, Papa A, Günther S, Drosten C (2007) Virus detection and monitoring of viral load in Crimean-Congo hemorrhagic fever virus patients. Emerg Infect Dis 13(7):1097–100
Acknowledgments
We thank the Refik Saydam Hygiene Center of Ankara, Turkey for testing the serum samples.
Ethical approval
The study was approved by the Medical Ethics Committee of the Cumhuriyet University School of Medicine.
Funding
None.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bakır, M., Gözel, M.G., Köksal, İ. et al. Validation of a severity grading score (SGS) system for predicting the course of disease and mortality in patients with Crimean-Congo hemorrhagic fever (CCHF). Eur J Clin Microbiol Infect Dis 34, 325–330 (2015). https://doi.org/10.1007/s10096-014-2238-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2238-0