Abstract
Tularaemia has mainly been a sporadic disease in Norway. In 2011, 180 persons (3.7 per 100,000 population) were diagnosed with tularaemia. This article describes the epidemiological and clinical features of tularaemia cases during a year with exceptionally high tularaemia incidence. Data from the national reference laboratory for tularaemia combined with epidemiological data from the Norwegian Surveillance System for Communicable Diseases (MSIS) were used. The incidence of tularaemia varied greatly between counties, but almost every county was involved. The majority (77.8 %) of the cases were diagnosed during the autumn and winter months. The geographic distribution also showed seasonal patterns. Overall, oropharyngeal tularaemia (41.1 %) was the most common clinical presentation, followed by glandular (14.4 %), typhoidal (14.4 %), respiratory (13.3 %) and ulceroglandular (12.8 %) tularaemia. From January to April, oropharyngeal tularaemia dominated, from May to September, ulceroglandular tularaemia was most common, whereas from October to December, there was an almost even distribution between several clinical forms of tularaemia. Eighty-five (47.2 %) of all tularaemia cases were admitted to, or seen as outpatients in, hospitals. An unexpectedly high number (3.9 %) of the patients had positive blood culture with Francisella tularensis. The clinical manifestations of tularaemia in Norway in 2011 were diverse, and changing throughout the year. Classification was sometimes difficult due to uncharacteristic symptoms and unknown mode of transmission. In rodent years, tularaemia is an important differential diagnosis to keep in mind at all times of the year for a variety of clinical symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first human cases of tularaemia in Norway were described by Thjøtta in 1930 [1]. Several reports of tularaemia were published in the late 1960s and early 1970s [2–4], and since 1979, tularaemia has been notifiable to the Norwegian Surveillance System for Communicable Diseases (MSIS) at the Norwegian Institute of Public Health. Apart from an outbreak in 1984–1985, affecting 57 people in central Norway, only 0–12 cases of tularaemia were reported annually from the whole country in the period 1977–2001 (http://www.msis.no/) [5, 6]. From 2002 onwards, there has been an increase in the number of cases, with 13–66 cases reported each year, partly due to several small outbreaks in central and northern Norway [7–9]. In 2011, a higher number of tularaemia cases were reported than in any previous year, with high numbers in some areas where hardly any tularaemia cases had been reported before. In 2012 and 2013, 50 and 28 tularaemia cases were reported, respectively. The aim of this study was to describe the nationwide increase in the incidence of tularaemia in Norway in 2011, with a focus on the epidemiological and clinical characteristics of the tularaemia cases.
Methods
Microbiological investigation
Most tularaemia cases were diagnosed at the national reference laboratory for tularaemia at St. Olavs Hospital, Trondheim. This is the only laboratory that offers serological methods, polymerase chain reaction (PCR) and culture for the diagnosis of human tularaemia. In addition, some cases were diagnosed by culture at other laboratories. Cultures from clinical samples suspected or confirmed at the primary laboratory as Francisella tularensis were submitted to the reference laboratory for confirmation. At the national reference laboratory, in-house methods for the micro-agglutination (MA) test and immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) were used for the serological diagnosis of tularaemia, with the ELISA test mainly used as support for the agglutination test in doubtful or early cases of tularaemia before a definite diagnosis could be made. The MA test uses formalin-killed F. tularensis bacteria isolated from a dead hare, and the ELISA test uses outer membrane antigens as described by Bevanger et al. [5]. A cut-off titre of ≥16 was used to define a positive MA test result, and a titre of ≥128 as a high titre interpreted as present disease if linked to clinical symptoms and/or epidemiological information of tularaemia. The ELISA lower cut-off was calculated using 1.5× the mean optical density (OD) values of pooled human sera (PHS) from 30 blood donors. Culture was performed using standard bacteriology media, including cystine heart agar with and without antibiotics whenever tularaemia was suspected, and incubated in 5 % CO2 at 35 °C for up to 10 days. A real-time TaqMan PCR was used for the detection of F. tularensis both directly on DNA extracts from patient samples other than serum and on bacterial cultures. Primers and probes (Table 1) for this PCR were constructed based on aligned 407-bp sequenced amplicons of a conventional PCR targeting the tul4 gene described by Sjöstedt et al. [10] from 26 F. tularensis strains. Bacterial cultures suspected to be F. tularensis were confirmed by PCR, and exclusion of F. tularensis subspecies tularensis was done by a real-time PCR targeting the pdpD gene, as described by Tomaso et al. [11].
Case definition and classification
Tularaemia cases were reported to the MSIS based on the case definitions for confirmed and presumptive cases in the World Health Organization (WHO) guidelines on tularaemia [12]. The classification of tularaemia was done according to the same WHO guidelines based on clinical information and information regarding the transmission mode available from the physician request form, from phone contact with the referring physician and from the MSIS.
Demographic data for Norway as of 1st January 2011 for population by county, municipality, age and gender were derived from the Statistical Yearbook of Norway 2011, Statistics Norway (https://www.ssb.no/a/en/histstat/aarbok/2011_en.pdf). Data on geographic distribution were based on information on where disease transmission most likely occurred, or if such information was not available, on the municipality of residence of each case. The presentation of data in three time periods were chosen due to the knowledge of seasonal temperatures and climate in Norway, where May to September are the months in which tick or mosquito bites are most likely to occur.
Results
In 2011, 180 cases of tularaemia were reported to the MSIS, of which 133 (73.9 %) cases were diagnosed by serology alone: seroconversion (14 cases), four-fold or higher increase in agglutination titre (20 cases) or a single agglutination titre ≥128 (99 cases). Eighteen cases (10.0 %) were diagnosed by culture: blood (seven cases), wound specimens (nine cases) and tissue biopsies (two cases). Twenty-nine cases were diagnosed by PCR: oropharyngeal specimens (18 cases), wound specimens (four cases), respiratory specimens (four cases) and lymph node aspirates (three cases). Presumptive cases of tularaemia based on a single positive MA titre of ≥128 or PCR detection of F. tularensis DNA from clinical samples all had clinical and/or epidemiological information suggestive of tularaemia. Eight of the culture-positive cases and 24 of the 29 PCR-positive cases were also confirmed by serology. Of the 18 culture-positive cases, all were confirmed as F. tularensis by the tul4 gene PCR, and to not be subspecies tularensis by PCR for the pdpD gene.
The time from symptom debut to the time of diagnosis was reported to be approximately 1 week for nine cases, 2–3 weeks for 52 of the cases, 4–5 weeks for 52 of the cases and more than 6 weeks for 33 of the cases. For 57 cases, information regarding the time between symptom debut and diagnosis was not stated or insufficient. For 14 of the 18 culture-positive cases, the sample was taken from 1 to 3 weeks after the onset of symptoms, while for the remaining four cases, the time interval before sampling was not stated.
Descriptive epidemiology
The 180 cases of confirmed or presumptive tularaemia gives a case rate of 3.7 per 100,000 population. The gender distribution was 111 men (61.7 %) and 69 women, with a male to female ratio of 1.6:1. All age groups were affected (range 1.5–88 years), with an age and gender distribution as shown in Fig. 1.
Tularaemia was reported from all parts of the country, with cases reported from 85 of 430 municipalities in 18 of 19 Norwegian counties. For 16 tularaemia cases, there was information indicating that the location for transmission was different from their residing municipality. Seven of these were infected within their residing county, seven in a different Norwegian county and two might have been infected outside Norway (Sweden and Germany, respectively). The incidence varied greatly between counties, from 0 to 62.7 per 100,000 population. The highest incidence rates were recorded in the counties Finnmark (62.7 per 100,000 population), Nord-Trøndelag (15.1 per 100,000 population) and Sør-Trøndelag (14.3 per 100,000 population). Within counties, the incidence rate varied even more between different municipalities, from 0 to 427 per 100,000 population. From the northernmost county of Finnmark, which is the least densely populated area in Norway (73,400 inhabitants, 1.5 % of the total population) and from which only a total of five cases had been reported previously, 46 cases of tularaemia were reported in 2011. Of the 180 tularaemia cases, 133 (73.9 %) were diagnosed in the five northernmost counties, covering only 23.3 % of the population. Figure 2 shows the incidence rate of tularaemia in different counties.
Seasonal patterns for the geographic distribution of the tularaemia cases were as shown in Fig. 3. One hundred and forty (77 %) of the cases were diagnosed during winter, early spring and late autumn (January to April and October to December). From January to April, 57 cases were diagnosed, 51 of whom were from four counties in central Norway. During the summer and early autumn months, 40 sporadic tularaemia cases were diagnosed all across the country, with 16 of 19 counties affected. From October to December, 83 cases were diagnosed, with a particularly high number in the northernmost county, Finnmark, with as many as 44 cases being diagnosed from October to December.
Clinical presentation varied with season, as seen in Table 2, and transmission mode varied by clinical presentation, as seen in Table 3. Oropharyngeal tularaemia was the most common type overall, with 74 cases (41.1 % of the total). There was an almost equal number of ulceroglandular, glandular, respiratory and typhoidal tularaemia cases, but with seasonal differences. Forty-five of the oropharyngeal cases occurred from January to April, constituting 79.5 % of all tularaemia cases in this time period. Part of this has previously been reported as an outbreak [13]. In the periods May to September and October to December, all clinical forms were seen and were more evenly distributed than in January to April. However, ulceroglandular forms were mainly seen in May to September (13/23) and respiratory (16/24) and typhoidal (20/26) forms were mainly seen in October to December. Glandular forms were uncommon in January to April (2/26) compared to the other months.
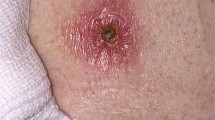
Sixty-five (87.8 %) of all the oropharyngeal cases had been drinking water from either a private well (n = 58) or an open stream (n = 7), and 95 % (n = 43) of the oropharyngeal cases from January to April reported the use of private wells. Among the 11 oropharyngeal cases from May to September, all reported drinking water from a private well, lake or stream as the likely mode of transmission. For the 18 oropharyngeal cases during October to December, 11 reported drinking water from a private well or from a stream as the transmission mode, four had an unknown transmission mode and three reported direct contact with lemmings as the likely transmission mode, probably by the hand-to-mouth route. Among the oropharyngeal cases, 63 presented with characteristic symptoms of sore throat, fever and swollen cervical lymph nodes, often unilateral. Among the patients classified as having oropharyngeal tularaemia, some had not noticed symptoms of tonsillitis or pharyngitis before developing cervical lymph node enlargement. These cases were classified as oropharyngeal tularaemia due to drinking water being the likely source of infection, and the fact that there was no possibility for transmission by mosquitoes or ticks at that particular time of the year. The remaining 11 oropharyngeal cases described uncharacteristic symptoms, such as fever, headache, diarrhoea and vomiting, but were still classified as oropharyngeal tularaemia due to a strong link to water as the likely source of infection. Filtrated water samples from three of the private wells used for drinking were positive for F. tularensis DNA by PCR.
Among the 23 ulceroglandular tularaemia cases, 13 were diagnosed during May to September, and nine from October to December. Among the cases from May to September, 12 of 13 were due to insect bites (three tick bites, three mosquito bites and six unspecified insect bites), whereas only one of the cases from October to December was due to an insect bite (unspecified). This sample was taken early in October and from a patient in the southern part of Norway. Four of the ulceroglandular cases from October to December were due to direct contact with an infected animal, and three of the four cases with unknown transmission mode were suspected to be linked to hunting or trekking activities. The single case of ulceroglandular tularaemia during January to April was due to direct contact with an infected animal.
For glandular tularaemia, 24 of the 26 cases occurred from May to December. The location of lymph node involvement was diverse, with the majority located in the groin (n = 6), axilla (n = 6) or collum (n = 9). Twenty-four of 26 cases with the typhoidal form occurred from May to December. The clinical presentation was dominated by fever, headache, myalgia, arthralgia and/or malaise, but with no organ-specific symptoms or signs. Among the 24 respiratory cases, the clinical information was diverse. Eleven cases were reported to have clinical pneumonia, and 13 had hilar lymphadenopathy and/or lung infiltrates, including five of those with clinical pneumonia. Four cases were diagnosed during investigation for suspected malignant disease based on radiological findings in patients with long-standing respiratory tract infection or fever. The remaining cases were classified as respiratory tularaemia due to inhalation as the likely transmission mode and symptoms of respiratory tract infection, such as cough, fever and dyspnoea.
For three cases, the type of tularaemia could not be determined due to the lack of information, and three cases diagnosed during outbreak investigation were asymptomatic. Two of these were from the same household and used the same water source as other tularaemia cases. A definite mode of transmission could not be established based on the available information for seven (12.3 %), 60 (72.3 %) and 13 (32.5 %) cases from the three time periods January to April, May to September and October to December, respectively. An unknown mode of transmission was the most common for typhoidal, respiratory and glandular forms of tularaemia, with 24, 21 and 20 cases of unknown transmission mode each, respectively. However, among 50 of these 80 cases, an association was reported to work or leisure activities associated with increased risk of being infected with tularaemia, such as hunting, trekking, farming and forest work (Table 3).
The morbidity of tularaemia infection was significant, with 57 (31.7 %) of the 180 cases admitted to hospital. Respiratory cases of tularaemia had the highest proportion of hospitalised patients, with 20 of 24 cases being treated as inpatients. In addition, 28 (15.6 %) were seen in a hospital as outpatients. Seven cases (3.9 %) had positive blood cultures (three respiratory cases, two typhoidal cases, one glandular case and one oropharyngeal case). Seventy-four of the patients had recovered clinically when the MSIS was notified by the physician, and 51 were still ill. However, these data have not been collected systematically, and do only reflect the situation at the time when the physician sent their notification to the national authorities. It is, therefore, possible that patients who were still symptomatic had their notification sent earlier in the course of the disease than those who had recovered. For 55 of the cases, there was no information available about the course of disease.
Control measures
The use of private wells is still common in rural areas of Norway, although the exact data on such use are not available. The mechanism on how such wells were contaminated is unknown, but was probably due to the flow of contaminated surface water into the well, or rodents entering unsecured wells. In March 2011, the Norwegian Food Authorities and the Norwegian Institute of Public Health issued advice to the population on not to consume water from streams or private wells that might be contaminated without prior boiling or disinfection, and for every well owner to undertake measures to prevent rodents or contaminated surface water from entering the well to reduce the risk of tularaemia infections.
Discussion
The highest number of tularaemia cases reported so far in Norway occurred in 2011. The lemming density in Norway that year was the largest, both in magnitude and geographic distribution since 1969/1970, reaching very high densities in some locations (Rolf Ims, Department of Arctic and Marine Biology, University of Tromsø, Norway, personal communication). This is the most probable explanation for the high number of tularaemia cases, although there was also a peak this year in other small rodents that might be implicated in tularaemia transmission, like the grey-sided vole, field vole, tundra vole and bank vole. However, when lemming populations erupt, they often do so more steeply than vole populations, perhaps due to a more favourable adaptation to cold climate with breeding during the winter season [14]. Omland et al. have described a relationship between human cases of tularaemia and the population density of small rodents like the Norwegian lemming (Lemmus lemmus) [15]. Two of the more recently described smaller Norwegian outbreaks of tularaemia [7, 9] also coincided with a lemming outbreak in northern Fennoscandia in 2006–2007 [14]. Central Norway has been among the areas with the highest number of tularaemia cases in Norway for many years, and outbreaks have occurred on several occasions previously, but often linked to just one common water source [6, 8, 9]. What was unusual in 2011 was the large diversity of geographic locations both within central Norway during January to April and for the whole country during the rest of the year, and particularly the striking increase in Finnmark during late 2011. Apart from local differences in breeding conditions and seasonal dynamics for lemmings and other rodent populations, any explanation for this has not been possible to identify. Another aspect that might have contributed to the high number of tularaemia cases diagnosed in 2011 was increased awareness of tularaemia in the general population and among health care workers due to considerable focus on lemmings and tularaemia in the Norwegian mass media. This awareness may have led patients with uncharacteristic symptoms to seek medical attention, and the health care worker to request more tests for tularaemia. There was no change in methodology at the reference laboratory that could explain the high number of diagnosed cases during that year.
The culture of F. tularensis has traditionally been regarded as potentially hazardous, with risk of laboratory infection, and to have inherent low sensitivity for the diagnosis of ulceroglandular tularaemia [16]. For those reasons, culture is often not attempted by many laboratories. Little information exists regarding the sensitivity of culture in oropharyngeal tularaemia, but based on reports from outbreaks where culture has been attempted, the sensitivity of culture in this form of tularaemia may be low [17]. Among our 18 PCR-positive oropharyngeal cases of tularaemia, 17 specimens were also cultured, all if which were negative. Eight of these were taken approximately 2 to 3 weeks after symptom onset, five were taken approximately 5 weeks after symptom onset and for five cases, information was missing. Our experience is, therefore, that PCR, with its potential to secure a rapid diagnosis, is of central importance for establishing the diagnosis of oropharyngeal tularaemia in conjunction with serology, while culture has low sensitivity for this diagnosis. According to a review by Karagöz et al. [18], F. tularensis is rarely cultured from blood. They found that positive blood cultures with F. tularensis have been reported in only 28 cases from 1977 to 2011, and mainly from the USA. Seven blood culture isolates with F. tularensis in one year from Norway, which has only five million inhabitants, is, therefore, a surprisingly high number.
While the age and gender distribution among the tularaemia cases in Norway is similar to what is reported from other European countries, Norway seems to have a different seasonal distribution of tularaemia (Fig. 4), with more cases reported during the winter months, both in 2011 and in other recent years [19]. In Europe, tularaemia has largely been reported to be a seasonal disease, with most cases being diagnosed during the summer and early autumn months (July to September), often related to insect bites [16, 19]. Also in Norway, the majority of ulceroglandular cases in 2011 were diagnosed during the summer months. Of the 17 ulceroglandular and glandular cases related to an insect bite, 14 were diagnosed during the period July to September. Thirteen of these cases were from the southern regions of Norway, and the four cases from Nord-Trøndelag and Nordland counties were all diagnosed during July and August, corresponding to the time of year when mosquito bite is likely to occur in these regions. In Sweden, ulceroglandular tularaemia is more common than in Norway, occurs mainly during summer and is related to mosquito bites, mosquito density and closeness to water [20]. Why mosquitoes and ticks seem to play a less important role in disease transmission in Norway is not clear. It is, however, a fact that the majority of tularaemia cases in Norway in 2011 were diagnosed during a time of the year when ticks and mosquitoes are not active, or were from inland or northern regions of the country, where there are no or a low number of ticks compared to the western and southern coastal areas of Norway.
For reasons we do not know for certain, oropharyngeal tularaemia seems to play a far more important role in Norway than what is documented from many other European countries [6, 19]. Many of the Norwegian cases of tularaemia in 2011 were related to contaminated water, and waterborne tularaemia outbreaks have been described by others [21]. Snow melting and contamination of unsecure private wells with F. tularensis-infected carcasses of, or faeces from, small rodents may explain the high number of tularaemia linked to the use of private wells during January to April [13]. The public health advice issued in March may have reduced the spread of disease through this route later in 2011. Apart from the recovery of F. tularensis DNA in filtered water samples, and epidemiological information where up to seven persons drinking from the same water source were diagnosed with tularaemia, there is no definitive evidence for contaminated water being the transmission mode. The consumption of undercooked contaminated food is not very likely. Hand-to-mouth transmission after contact with animals or contaminated environment cannot be excluded as a possibility, particularly for children. This was also suspected in three of the cases from October to December. However, more oropharyngeal tularaemia among persons not using private wells might have been suspected if these were common transmission modes.
Respiratory tularaemia, together with ulceroglandular tularaemia, has been the predominating form of tularaemia in Finland and Sweden, often linked to farming [16]. In Norway, the transmission mode was unknown for 21 of the 24 respiratory cases in 2011, but some were probably linked to the inhalation of dust contaminated with bacteria during farm or forest-related work, like lawn mowing, harvesting and rehabilitation work on old farm buildings. Seven of these cases reported direct or indirect contact with live or dead lemmings as a possible mode of transmission, as huge numbers had been present in their environment, with possible inhalation of bacteria. The use of personal protective equipment such as gloves and respiratory protection should be considered when performing activities as mentioned above in areas with high numbers of rodents.
2011 showed an unusually high number and geographic spread among the tularaemia cases in Norway. The clinical manifestations were diverse, and changed throughout the year. A considerable proportion of the patients presented with significant morbidity, with almost 50 % being in contact with their local hospital on one or several occasions. Some described a clinical picture which involved more than one organ system, and were, therefore, difficult to classify according the WHO guidelines [12]. Almost 50 % had a greater than 4-week interval between symptom onset and time of diagnosis, making it difficult to establish with certainty both the transmission mode and where the disease had been contracted. Serology and PCR were the most important diagnostic tools, although an unexpectedly high number of cases had recovery of F. tularensis from blood culture. To diagnose tularaemia remains a challenge when the clinical presentation is uncharacteristic and the time of the year uncommon for tularaemia. It is, however, an important diagnosis to consider at all times of the year in cases of, for instance, protracted fever, cough or lymph node enlargement, particularly if there is an epidemiological link to a rodent year, use of private wells, or work or leisure activities that may be associated with an increased risk of contracting tularaemia.
References
Thjøtta T (1930) Tre tilfelle av tularemia. En i Norge hittil ikke erkjent sykdom. Nor Mag Lægevidensk 91:224–236
Henriksen SD, Holt G, Müller G (1965) En tularemiepidemi i Kviteseid. Nord Med 73(2):43
Mair IW, Natvig K, Johannessen TA (1973) Otolaryngological manifestations of tularemia. Arch Otolaryngol 98:156–158
Haug RH, Pearson AD (1972) Human infections with Francisella tularensis in Norway. Development of a serological screening test. Acta Pathol Microbiol Scand B Microbiol Immunol 80(2):273–280
Bevanger L, Maeland JA, Naess AI (1988) Agglutinins and antibodies to Francisella tularensis outer membrane antigens in the early diagnosis of disease during an outbreak of tularemia. J Clin Microbiol 26(3):433–437
Brantsæter AB (2004) Twenty-five years of tularaemia in Norway, 1978–2002. In: Proceedings of the 14th European congress of clinical microbiology and infectious diseases, Prague, Czech Republic, May 2004. European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Abstract number: 10.1111/j.1198-743X.2004.902_o142.x
Brantsaeter AB, Krogh T, Radtke A, Nygard K (2007) Tularaemia outbreak in northern Norway. Euro Surveill 12(13):E070329.2
Rike HF, Vigerust A, Bergh K (2003) Vannbårent utbrudd av tularemia (harepest) i Midtre Gauldal. [A waterborne outbreak of tularaemia in Midtre-Gauldal.] Norwegian Institute of Public Health. Norwegian. Available from: http://www.fhi.no/eway/default.aspx?pid=233&trg=MainLeft_5669&MainLeft_5669=5544:27208::0:5667:1:::0:0
Melien P, Holsdal RE (2008) Tularemi i Meldal—en vanskelig diagnose? [Tularaemia in Meldal—a difficult diagnosis?] Norwegian Institute of Public Health. Norwegian. Available from: http://www.fhi.no/eway/default.aspx?pid=233&trg=Area_5626&MainArea_5661=5619:0:15,4427:1:0:0:::0:0&MainLeft_5619=5626:68396::1:5625:1:::0:0&Area_5626=5544:68400::1:5628:1:::0:0
Sjöstedt A, Eriksson U, Berglund L, Tärnvik A (1997) Detection of Francisella tularensis in ulcers of patients with tularemia by PCR. J Clin Microbiol 35(5):1045–1048
Tomaso H, Scholz HC, Neubauer H, Al Dahouk S, Seibold E, Landt O, Forsman M, Splettstoesser WD (2007) Real-time PCR using hybridization probes for the rapid and specific identification of Francisella tularensis subspecies tularensis. Mol Cell Probes 21:12–16
World Health Organization (WHO) Epidemic and Pandemic Alert and Response (2007) WHO guidelines on tularaemia. WHO, Geneva. Available from: http://www.who.int/csr/resources/publications/WHO_CDS_EPR_2007_7.pdf
Larssen KW, Afset JE, Heier BT, Krogh T, Handeland K, Vikøren T, Bergh K (2011) Outbreak of tularaemia in central Norway, January to March 2011. Euro Surveill 16(13):19828
Ims RA, Yoccoz NG, Killengreen ST (2011) Determinants of lemming outbreaks. Proc Natl Acad Sci U S A 108(5):1970–1974
Oaaland T, Christiansen E, Jonsson B, Kapperud G, Wiger R (1977) A survey of tularemia in wild mammals from fennoscandia. J Wildl Dis 13:393–399
Tärnvik A, Priebe HS, Grunow R (2004) Tularaemia in Europe: an epidemiological overview. Scand J Infect Dis 36:350–355
Gürcan S, Karabay O, Karadenizli A, Karagöl Ç, Kantardjiev T, Ivanov IN (2008) Characteristics of the Turkish isolates of Francisella tularensis. Jpn J Infect Dis 61:223–225
Karagöz S, Kiliç S, Berk E, Uzel A, Çelebi B, Çomoğlu Ş, Karagöz A, Akyar I, Can S (2013) Francisella tularensis bacteremia: report of two cases and review of the literature. New Microbiol 36:315–323
European Centre for Disease Prevention and Control (ECDC) (2013) Annual epidemiological report 2012. Reporting on 2010 surveillance data and 2011 epidemic intelligence data. ECDC, Stockholm
Rydén P, Björk R, Schäfer ML, Lundström JO, Petersén B, Lindblom A, Forsman M, Sjöstedt A, Johansson A (2012) Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis 205:297–304
Willke A, Meric M, Grunow R, Sayan M, Finke EJ, Splettstößer W, Seibold E, Erdoğan S, Ergonul O, Yumuk Z, Gedikoglu S (2009) An outbreak of oropharyngeal tularaemia linked to natural spring water. J Med Microbiol 58:112–116
Acknowledgements
We would like to thank Kirsten Konsmo, Department of Infectious Disease Epidemiology, Norwegian Institute of Public Health, for the technical support with the maps, and Frode Width Gran, Department of Medical Microbiology, St. Olavs Hospital, for the technical support with Fig. 1.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larssen, K.W., Bergh, K., Heier, B.T. et al. All-time high tularaemia incidence in Norway in 2011: report from the national surveillance. Eur J Clin Microbiol Infect Dis 33, 1919–1926 (2014). https://doi.org/10.1007/s10096-014-2163-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2163-2