Abstract
Pneumocystis pneumonia (PcP) is a major HIV-related illness caused by Pneumocystis jirovecii. Definitive diagnosis of PcP requires microscopic detection of P. jirovecii in pulmonary specimens. The objective of this study was to evaluate the usefulness of two serum markers in the diagnosis of PcP. Serum levels of (1–3)-beta-d-glucan (BG) and lactate dehydrogenase (LDH) were investigated in 100 HIV-positive adult patients and 50 healthy blood donors. PcP cases were confirmed using indirect immunofluorescence with monoclonal anti-Pneumocystis antibodies and nested-PCR to amplify the large subunit mitochondrial rRNA gene of P. jirovecii in pulmonary specimens. BG and LDH levels in serum were measured using quantitative microplate-based assays. BG and LDH positive sera were statistically associated with PcP cases (P ≤ 0.001). Sensitivity, specificity, positive/negative predictive values (PPV/NPV), and positive/negative likelihood ratios (PLR/NLR) were 91.3 %, 61.3 %, 85.1 %, 79.2 %, 2.359, and 0.142, respectively, for the BG kit assay, and 91.3 %, 35.5 %, 75.9 %, 64.7 %, 1.415 and 0.245, respectively, for the LDH test. Serologic markers levels combined with the clinical diagnostic criteria for PcP were evaluated for their usefulness in diagnosis of PcP. The most promising cutoff levels for diagnosis of PcP were determined to be 400 pg/ml of BG and 350 U/l of LDH, which combined with clinical data presented 92.8 % sensitivity, 83.9 % specificity, 92.8 % PPV, 83.9 % NPV, 5.764 PLR and 0.086 NLR (P < 0.001). This study confirmed that BG is a reliable indicator for detecting P. jirovecii infection. The combination between BG/LDH levels and clinical data is a promising alternative approach for PcP diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pneumocystis jirovecii is an opportunistic pathogen capable of causing fatal interstitial pneumonia in immunocompromised patients. Clinical diagnosis is complex because no clinical symptoms, radiologic features, or gasometric data are specific to Pneumocystis pneumonia (PcP) [1–5]. Since P. jirovecii cannot be cultured reliably, non-culturing methods are used for diagnosis of the disease, including direct cytochemical staining, immunofluorescent staining with monoclonal antibodies (IF), and molecular methods such as PCR [6–13].
Normally, pulmonary specimens obtained by invasive techniques, such as bronchoalveolar lavage, are used for diagnosis of PcP. However, bronchoalveolar lavages (BAL) are not easy to perform or repeat on PcP patients, particularly on those with respiratory failure or with AIDS, or on children. Although induced sputum (IS) specimens, of less invasive nature than BAL, can also be used, they have to be collected in infectious diseases clinics that carefully control sputum induction. These methods are too expensive and difficult to implement in developing regions of the world. Also, asymptomatic carriers that are an important epidemiological group are not easily subjected to these methodologies [1–4, 8, 12, 14, 15]. Thus, an improved P. jirovecii detection method using a readily available, less expensive and minimally invasive sample, such as blood, is desired [11, 15–24].
Recent data showed that measurement of (1–3)-beta-d-glucan (BG) (cell wall component of P. jirovecii cysts) in the serum is a promising tool for diagnosis of PcP [25–29]. BG can be detected and measured quantitatively in patients’ sera, and a correlation between BG levels and diagnosis or severity of organic mycosis is recognized [30, 31]. Studies also suggest that it is possible to distinguish between PcP and other fungal infections in immunocompromised patients based on serum BG levels and that repeated checks of BG levels in serum may be useful for evaluation of disease progression and therapeutic efficacy [25, 27–30]. The measurement of BG levels in serum as a diagnostic indicator of PcP has yielded a wide range of results, with a sensitivity of 90–100 % and a specificity of 65–100 % [16, 18, 20, 23, 26, 28, 31]. Several studies have found that serum levels of lactate dehydrogenase (LDH) (released from cells upon damage of their cytoplasmic membrane) are increased in PcP patients, probably due to lung injury caused by the pathogen. The level of LDH as a marker of PcP has been shown to have a 78–100 % sensitivity and 45–78 % specificity [19, 23, 24, 32].
The present study aimed to: (1) investigate possible associations between BG/LDH levels and patients’ clinical parameters, and (2) validate BG and LDH as reliable serum indicators of P. jirovecii infection and demonstrate the usefulness of their association in the diagnosis of PcP.
Patients and methods
Subjects
A total of 150 participants (100 patients and 50 healthy blood donors) were included in this study. One hundred pulmonary specimens (65 IS and 35 BAL fluids) and sera were obtained from the 100 HIV-positive adult patients with respiratory symptoms, living in Lisbon, Portugal. These samples were collected between 1998 and 2011 for diagnostic purpose before patients start their anti-P. jirovecii therapeutic regimen with trimethoprim-sulfamethoxazole (TMP-SMZ). Patients mean age was 38 years (ranged 20–68 years), and most (77 %) were men. Fifty sera were collected from blood donors and used as a negative control to validate the experimental assays. These samples were randomly selected from a group of healthy individuals (weight equal to or greater than 50 kg and aged between 18 and 65). The present study had the approval of the Institutional Review Boards/Ethical Committees from the involved institutions.
Detection of P. jirovecii organisms was performed in pulmonary specimens by both indirect immunofluorescence with anti-P. jirovecii monoclonal antibodies (IF) (MonoFluoTM kit P. jirovecii; Bio-Rad, France) and nested-PCR directed to the large subunit mitochondrial rRNA gene of P. jirovecii. DNA extraction was performed using the Mini-BeadBeater/guanidinium thiocyanate-silica method [7, 13, 33, 34]. A clinical diagnosis of PcP was considered when at least two of the following variables were present: symptoms such as unproductive cough, fever, and dyspnea; arterial partial pressure of oxygen (PaO2) lower than 65 mmHg; and chest radiographs presenting fine bilateral, perihilar interstitial shadowing. Proven PcP cases were defined as HIV-positive patients fulfilling clinical diagnostic criteria for PcP with positive IF and/or nested-PCR. Undiagnosed PcP or atypical PcP cases were defined as HIV-positive patients not fulfilling clinical diagnostic criteria for PcP with positive IF and nested-PCR. Colonized patients or subclinical infections were described as HIV-positive patients not fulfilling clinical diagnostic criteria for PcP with negative P. jirovecii IF and positive nested-PCR [1, 5, 34, 35]. Data on CD4+ T lymphocyte counts and anti-P. jirovecii prophylaxis were also available. P. jirovecii burden was estimated (using the semi-quantitative method of IF) in the patients incorrectly diagnosed by BG and LDH assays, e.g. low P. jirovecii burden (no cysts identified by IF, but positive by nested-PCR), moderate P. jirovecii burden (one to three cysts in 30 fields at × 1000 by IF) [33, 34].
BG and LDH levels in serum
The serum BG levels were determined by using a quantitative microplate assay based upon the kinetics of Limulus Amebocyte Lysate (LAL) pathway (Fungitell Assay, Associates of Cape Cod, MA, USA) [18, 29, 30, 36]. The reference values established for fungal infections and the baseline limits of the method are as follows: negative, <60 pg/ml; indeterminate, 60–79 pg/ml; positive, ≥80 pg/ml; upper limit, ≤500 pg/ml; and lower limit, ≥31 pg/ml [16, 25, 28]. LDH levels were determined using the quantitative Lactate Dehydrogenase Assay Kit (BioVision, CA, USA), in which LDH reduces nicotinamide adenine dinucleotide (NAD). The reduced form (NADH) interacts with a specific probe to produce colour. LDH levels in healthy persons are usually between 100 and 350 U/l [19, 22, 24]. All samples were tested in duplicate for each serologic marker. Both assays were performed in the NanoQuant Infinite M200 Pro (TECAN, Switzerland).
Data analysis
Chi-square test and Fisher’s exact test were used to investigate the association between qualitative variables. Mann–Whitney U test was used to investigate the differences between the distribution of BG and LDH levels in serum samples across distinct categories of patients (e.g. patients with and without PcP). The Spearman rank-order correlation test was applied to study the relationship between BG and LDH levels. Receiver operating characteristic (ROC) curves, sensitivity (the percent of patients with PcP correctly identified by the BG or LDH test), specificity (the percent of patients without PcP correctly identified by the BG or LDH test), positive predictive values (PPV) (the percent of PcP patients with a positive BG or LDH test who did have PcP), negative predictive values (NPV) (the percent of PcP patients with a negative BG or LDH test who did not have PcP) and the positive/negative likelihood ratios (PLR/NLR) (the ratio of the probabilities that the test will be positive/negative in cases with PcP versus those without PcP) were calculated to assess the utility of BG and LDH levels for PcP diagnosis [20]. SPSS v.20.0 software (SPSS Inc., Chicago, IL, USA) and the software R (http://www.r-project.org) were used to investigate associations at a significance level of 0.05.
Results
The laboratory and clinical parameters of the patients and blood donors involved in the study are summarized in Table 1. Of the 100 patients studied, 69 were diagnosed with PcP (three atypical PcP cases, not fulfilling clinical diagnostic criteria for PcP), and 31 were PcP negative. Among the PcP negative cases, 12 (39 %) were colonized patients and 19 (61 %) were patients with other pulmonary diseases with no detectable P. jirovecii organisms.
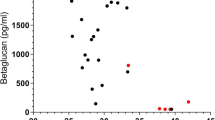
The BG and LDH levels in patients with and without PcP and in the blood donors are depicted in Fig. 1 and Table 2. The ROC curves for BG and LDH are shown in Fig. 2. The Spearman rank-order correlation analysis revealed a statistically significant correlation between the serum levels of these two markers in the population studied (P < 000.1, R2 = 0.106). To determine the optimal cutoff limits of BG and LDH for the diagnosis of PcP, three different levels of each biomarker were analyzed (80 pg/ml, 100 pg/ml, and 400 pg/ml for BG; 350 U/l, 550 U/l, and 1000 U/l for LDH). The most promising results were obtained using the cutoff limits of 100 pg/ml BG and 550 U/l LDH. The results are shown in Table 3.
Graphic representation of the distribution of (1–3)-beta-d-glucan (BG) and lactate dehydrogenase (LDH) values in patients with and without PcP and in the blood donors (negative control group). The box-and-whisker plots show the 25th and 75th percentiles, the median (horizontal line within the boxes), and the 10th and 90th percentiles (whiskers). The baseline limits for BG detection (lower limit 31 pg/ml; and upper limit 500 pg/ml) were considered. Serum levels of BG and LDH were significantly higher in PcP patients (P < 0.001)
ROC curves for (1–3)-beta-d-glucan (BG) and lactate dehydrogenase (LDH) assays. For the BG assay, the area under the curve (AUC) was 86.4 % with a 95 % confidence interval of 78.8–94.0 %. For LDH, the AUC was 72.7 % with a 95 % confidence interval of 61.7–83.7 %. The AUC obtained for the BG assay was significantly greater than the AUC from the LDH test (P=0.01), suggesting that BG is a more reliable indicator for detecting P. jirovecii infection than LDH
The diagnostic value of the combination of BG and LDH levels and clinical data for PcP diagnosis was then evaluated (Table 4). A definitive diagnosis of PcP was considered when at least two of the following variables were present: fulfilling clinical diagnostic criteria for PcP, BG serum levels higher than the established cutoff limits (80 pg/ml; 100 pg/ml; 400 pg/ml), and LDH serum levels higher than the LDH cutoff limits (350 U/l; 550 U/l; 1000 U/l). The most promising cutoff levels of this alternative diagnostic procedure were determined to be 400 pg/ml of BG and 350 U/l of LDH combined with clinical data of PcP; this combination was found to have a 92.8 % sensitivity and 83.9 % specificity (Table 4). Of the 100 patients studied, 90 were correctly diagnosed with this procedure. Five PcP patients were undiagnosed using this method. Among these, two had atypical PcP presentation, not fulfilling clinical diagnostic criteria for PcP, two demonstrated low P. jirovecii burden and were negative for both markers, and one fulfilled clinical diagnostic criteria for PcP but was negative for both markers (BG 46.7 pg/ml and LDH 313 U/l). Five patients without P. jirovecii infection were positive by this procedure, including four patients with tuberculosis and one with bacterial pneumonia.
Discussion
In the present study, the usefulness of BG and LDH in association as serological markers for the diagnosis of PcP in HIV-positive patients was assessed. Although there have been several reports describing elevated serum levels of BG and LDH in PcP patients, the diagnostic value of these markers remains unclear for this pathology [2, 3, 11, 16, 25]. The results of this study confirmed that the serum level of BG is a reliable indicator of PcP and that the combination of serum BG and LDH levels and parameters of clinical diagnosis is a promising approach as an alternative procedure for PcP diagnosis.
Several studies indicate the usefulness of the BG test for diagnosis of PcP, but none of them determined the optimal cutoff limit of the test [16, 37]. Our results demonstrated that 100 pg/ml was an optimal cutoff limit for BG, when used alone. It had a sensitivity of 89.9 % and specificity of 71.0 %, better than those of the cutoff limits recommended by the manufacturer of the BG assay, considering the population studied (Table 3). Using the recommended cutoff value, we found that the BG assay had a 91.3 % sensitivity which is consistent with those of previous studies, where sensitivity ranges from 86 to 100 %. However, the 61.3 % specificity was slightly lower than the specificity reported by other investigators (ranging from 65 to 91 %) [20, 28]. Since it is not a species-specific marker, it is not surprising that the specificity level of the BG test was low. Our results showed that the median BG levels were the highest in PcP patients (270 pg/ml), followed by P. jirovecii colonized patients (67 pg/ml), patients with other pulmonary diseases (36 pg/ml), and healthy people (31.0 pg/ml) (Table 2). The BG kit assay yielded five false-negative and 11 false-positive results. Of the five false-negative cases, four had low P. jirovecii burden, and one had no apparent reason for the low BG levels. These BG false-negative cases may be in the early stage of infection in which few P. jirovecii cysts were destroyed, and thus the BG serum level is low. Of the 11 false-positive cases, five had P. jirovecii colonization. Among the remaining six cases, two had oropharyngeal candidiasis, and four showed clinical conditions suggestive of fungal infections. The median BG level of these six cases was 172.4 pg/ml, significantly lower than that (270 pg/ml) of patients with PcP. It is well known that invasive fungal infections such as candidiasis, aspergillosis, fusariosis, trichosporonosis and histoplasmosis may give rise to a positive BG test [27, 28, 30]. However, the serum BG levels of patients with these diseases are generally lower than those with PcP [16, 27, 38]. These data strongly suggest that the BG test may be helpful in distinguishing PcP from other fungal infections in immunocompromised patients. A recent meta-analysis also indicates that the diagnostic accuracy of the BG assay is high for PcP and moderate for invasive fungal infections, strengthening the hypothesis that this method may be used as a screening test for PcP [27]. Of the two cases with indeterminate BG results, one was a PcP patient with low P. jirovecii burden, and the other had P. jirovecii colonization. As shown previously [39, 40], P. jirovecii colonization may occur in patients with chronic respiratory disease (10–40 % in chronic obstructive disease patients), especially in those receiving corticosteroid therapy. The results from recent studies suggest that serum BG levels may be useful in distinguishing P. jirovecii colonization from PcP [38, 41–43]. Our results also indicate such possibility (Table 2).
The present study showed that the median LDH levels were the highest in PcP patients (707 U/l), followed by P. jirovecii colonized patients (474 U/l), patients with other pulmonary diseases (419 U/l), and healthy people (141 U/l). In contrast to BG, the analysis of LDH was not able to distinguish between colonization by P. jirovecii and other pulmonary syndromes because the median LDH levels in these two groups of patients were similar (Table 2). There is no definite LDH diagnostic cutoff value to discriminate PcP cases from other diseases or PcP patients from colonization cases. The LDH test with a 350 U/l cutoff limit had 91.3 % sensitivity and 35.5 % specificity (Table 3). This sensitivity value was consistent with those of previous studies where sensitivity ranges from 78 to 100 %, but the specificity value was slightly lower than those reported by other investigators (ranging from 47 to 78 %) [22, 24, 32]. Although increased levels of serum LDH are commonly seen in PcP patients, high LDH levels may also be caused by other lung infections and a variety of extrapulmonary disorders.
In this study, the LDH test with a 350 U/l cutoff value led to six false-negative and 20 false-positive cases. Of the six false-negative cases, three had a low P. jirovecii burden, probably a reflection of an early stage of infection with slight epithelial damage, and thus a low LDH serum level. The other three false-negative cases presented moderate P. jirovecii burden; however, their LDH levels were relatively close to the cutoff limit of 350 U/l (307 U/l, 311 U/l, 287 U/l). Because high serum levels of LDH are normally an indication of organ damage rather than specific diseases, the occurrence of 20 false-positive results was not surprising. Of these, 15 had other diseases such as tuberculosis, bacterial pneumonia, lymphoma, or chronic obstructive pulmonary disease, and the remaining five had no established infection at the time of sample collection, but were clinically suggestive of having pulmonary infections other than PcP. These results confirmed that serum LDH levels may serve as an adjunctive marker for PcP diagnosis, but should be interpreted with caution due to probable coexisting diseases or serum enzyme abnormalities.
The evaluation of the relationship between BG and LDH levels showed that these two serologic markers were positively correlated (Fig. 2). If used together, these markers may add additional reliability to the serological diagnosis of PcP. It is well known that the sole use of clinical data, chest X-ray, and gasometry tests cannot distinguish PcP from bacterial pneumonia or tuberculosis. However, these parameters are relevant for the presumptive diagnosis, which usually leads to laboratory examination of pulmonary specimens.
The present data propose an alternative method for complementary PcP diagnosis based on the association between clinical diagnostic criteria and serum BG and LDH levels. From the several combinations tested, the one composed by clinical diagnosis with BG cutoff 400 pg/ml and LDH cutoff 350 U/l was found to be the most promising approach as it had 92.8 % sensitivity and 83.9 % specificity for diagnosis of PcP (Table 4). Moreover, of the 100 patients included in the study, 90 were correctly diagnosed with this procedure with five false-positive and five false-negative results. Of the five false-negative cases, two did not fulfill clinical diagnostic criteria for PcP (atypical PcP cases) and were BG negative and LDH positive. Three fulfilled the clinical diagnostic criteria for PcP but were negative for both markers. Among these three cases, two presented with low P. jirovecii burden, which may account for the low BG and LDH contents in the blood, and one had no apparent reason for the negative result. However, it cannot be ruled out that the false-negative cases may be due to a recent infection in which P. jirovecii organism load was still low, and the infection has not caused elevated levels of BG and LDH. Of the five false-positive cases, all fulfilled the clinical diagnostic criteria for PcP and were BG negative and LDH positive. These five cases included four patients with tuberculosis and one with bacterial pneumonia. These results confirmed again that LDH levels are less reliable for PcP diagnosis. With this specific combination, the PLR was moderately high (5.764) and the NLR was very low (0.086). The PLR result reflects the fact that these markers (especially LDH) might be falsely positive in some patients without PcP, while the NLR result indicates that a negative test is much less likely to be observed in patients with PcP compared with patients without PcP.
The proposed method for PcP diagnosis based on the quantification of BG (P. jirovecii marker) and LDH (host marker) levels in serum of patients fulfilling the clinical diagnostic criteria for PcP may be used as an adjunctive noninvasive preliminary screening test to diagnose this disease. Although it will not replace the classic gold standard diagnostic procedures for PcP, such as cytochemical staining or IF in BAL or IS samples, it’s high sensitivity and moderate specificity may reduce the practice of empirical treatment of PcP, especially in developing countries, in patients with respiratory failure, or children in whom invasive procedures for specimen collection are not easy to perform. Nevertheless, the diagnostic usefulness of this serologic method remains to be confirmed in a larger cohort of patients with a variety of underlying diseases.
References
Barry SM, Johnson MA (2001) Pneumocystis carinii pneumonia: a review of current issues in diagnosis and management. HIV Med 2(2):123–132
Calderon EJ, Gutierrez-Rivero S, Durand-Joly I, Dei-Cas E (2010) Pneumocystis infection in humans: diagnosis and treatment. Expert Rev Anti Infect Ther 8(6):683–701
Huang L, Cattamanchi A, Davis JL et al (2011) International HIV-associated opportunistic pneumonias (IHOP) study; lung HIV study: HIV-associated Pneumocystis pneumonia. Proc Am Thorac Soc 8(3):294–300
Morris A, Lundgren JD, Masur H et al (2004) Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis 10(10):1713–1720
Walzer PD, Evans HE, Copas AJ, Edwards SG, Grant AD, Miller RF (2008) Early predictors of mortality from Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985–2006. Clin Infect Dis 46(4):625–633
Arcenas RC, Uhl JR, Buckwalter SP et al (2006) A real-time polymerase chain reaction assay for detection of Pneumocystis from bronchoalveolar lavage fluid. Diagn Microbiol Infect Dis 54(3):169–175
Esteves F, Gaspar J, De Sousa B, Antunes F, Mansinho K, Matos O (2011) Clinical relevance of multiple single-nucleotide polymorphisms in Pneumocystis jirovecii pneumonia: development of a multiplex PCR-single-base-extension methodology. J Clin Microbiol 49(5):1810–1815
Holten-Andersen W, Kolmos HJ (1989) Comparison of methenamine silver nitrate and Giemsa stain for detection of Pneumocystis carinii in bronchoalveolar lavage specimens from HIV infected patients. APMIS 97(8):745–747
Lautenschlager I, Lyytikainen O, Jokipii L et al (1996) Immunodetection of Pneumocystis carinii in bronchoalveolar lavage specimens compared with methenamine silver stain. J Clin Microbiol 34(3):728–730
Matos O, Lundgren B, Caldeira L et al (2000) Evaluation of two nested polymerase chain reactions for diagnosis of Pneumocystis carinii pneumonia in immunocompromised patients. Clin Microbiol Infect 6(3):149–151
Morris AM, Masur H (2011) A serologic test to diagnose Pneumocystis pneumonia: are we there yet? Clin Infect Dis 53(2):203–204
Turner D, Schwarz Y, Yust I (2003) Induced sputum for diagnosing Pneumocystis carinii pneumonia in HIV patients: new data, new issues. Eur Respir J 21(2):204–208
Wakefield AE, Pixley FJ, Banerji S et al (1990) Detection of Pneumocystis carinii with DNA amplification. Lancet 336(8713):451–453
Matos O, Costa MC, Correia I et al (2006) Pneumocystis jiroveci infection in immunocompetent patients with pulmonary disorders, in Portugal. Acta Med Port 19(2):121–126
Matos O, Lundgren B, Caldeira L et al (1999) Evaluation of a nested PCR for detection of Pneumocystis carinii in serum from immunocompromised patients. J Eukaryot Microbiol 46(5):104S–105S
Desmet S, Van Wijngaerden E, Maertens J et al (2009) Serum (1–3)-beta-D-glucan as a tool for diagnosis of Pneumocystis jirovecii pneumonia in patients with human immunodeficiency virus infection or hematological malignancy. J Clin Microbiol 47(12):3871–3874
Djawe K, Huang L, Daly KR et al (2010) Serum antibody levels to the Pneumocystis jirovecii major surface glycoprotein in the diagnosis of P. jirovecii pneumonia in HIV+ patients. PLoS ONE 5(12):e14259
Finkelman MA (2010) Pneumocystis jirovecii infection: Cell wall (1–3)-B-D-glucan biology and diagnostic utility. Crit Rev Microbiol 36(4):271–281
Fox GN (1993) Elevated LDH and Pneumocystis carinii pneumonia. Am Fam Physician 47(7):1579–1582
Karageorgopoulos DE, Qu JM, Korbila IP, Zhu YG, Vasileiou VA, Falagas ME et al (2013) Accuracy of beta-d-glucan for the diagnosis of Pneumocystis jirovecii pneumonia: a meta-analysis. Clin Microbiol Infect 19(1):39–49
Nakamura H, Tateyama M, Tasato D et al (2009) Clinical utility of serum beta-D-glucan and KL-6 levels in Pneumocystis jirovecii pneumonia. Intern Med 48(4):195–202
Quist J, Hill AR (1995) Serum lactate dehydrogenase (LDH) in Pneumocystis carinii pneumonia, tuberculosis, and bacterial pneumonia. Chest 108(2):415–418
Tasaka S, Hasegawa N, Kobayashi S et al (2007) Serum indicators for the diagnosis of Pneumocystis pneumonia. Chest 131(4):1173–1180
Vogel M, Weissgerber P, Goeppert B et al (2011) Accuracy of serum LDH elevation for the diagnosis of Pneumocystis jiroveci pneumonia. Swiss Med Wkly 141:w13184
de Boer MG, Gelinck LB, van Zelst BD et al (2011) β-D-glucan and S-adenosylmethionine serum levels for the diagnosis of Pneumocystis pneumonia in HIV-negative patients: a prospective study. J Infect 62(1):93–100
Held J, Koch MS, Reischl U, Danner T, Serr A et al (2011) Serum (1→3)-beta-D-glucan measurement as an early indicator of Pneumocystis jirovecii pneumonia and evaluation of its prognostic value. Clin Microbiol Infect 17(4):595–602
Onishi A, Sugiyama D, Kogata Y et al (2012) Diagnostic accuracy of serum 1,3-beta-D-glucan for Pneumocystis jiroveci pneumonia, invasive candidiasis, and invasive aspergillosis: systematic review and meta-analysis. J Clin Microbiol 50(1):7–15
Sax PE, Komarow L, Finkelman MA et al (2011) AIDS Clinical Trials Group Study A5164 Team: Blood (1→ 3)-{beta}-D-Glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis 53(2):197–202
Yasuoka A, Tachikawa N, Shimada K, Kimura S, Oka S (1996) (1→ 3) beta-D-glucan as a quantitative serological marker for Pneumocystis carinii pneumonia. Clin Diagn Lab Immunol 3(2):197–199
Odabasi Z, Mattiuzzi G, Estey E et al (2004) Beta-D-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin Infect Dis 39(2):199–205
Persat F, Ranque S, Derouin F, Michel-Nguyen A, Picot S, Sulahian A (2008) Contribution of the (1→3)-beta-D-glucan assay for diagnosis of invasive fungal infections. J Clin Microbiol 46(3):1009–1013
Grover SA, Coupal L, Suissa S et al (1992) The clinical utility of serum lactate dehydrogenase in diagnosing Pneumocystis carinii pneumonia among hospitalized AIDS patients. Clin Invest Med 15(4):309–317
Esteves F, Gaspar J, de Sousa B, Antunes F, Mansinho K, Matos O (2012) Pneumocystis jirovecii multilocus genotyping in pooled DNA samples: a new approach for clinical and epidemiological studies. Clin Microbiol Infect 18(6):E177–E184
Esteves F, Gaspar J, Marques T et al (2010) Identification of relevant single-nucleotide polymorphisms in Pneumocystis jirovecii: relationship with clinical data. Clin Microbiol Infect 16(7):878–884
Esteves F, Gaspar J, Tavares A et al (2010) Population structure of Pneumocystis jirovecii isolated from immunodeficiency virus-positive patients. Infect Genet Evol 10(2):192–199
Iwanaga S (1993) The limulus clotting reaction. Curr Opin Immunol 5(1):74–82
Kaplan JE, Hanson DL, Navin TR, Jones JL (1998) Risk factors for primary Pneumocystis carinii pneumonia in human immunodeficiency virus-infected adolescents and adults in the United States: reassessment of indications for chemoprophylaxis. J Infect Dis 178(4):1126–1132
Matsumura Y, Ito Y, Iinuma Y et al (2012) Quantitative real-time PCR and the (1→3)-beta-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect 18(6):591–597
Teramoto S, Sawaki D, Okada S, Ouchi Y (2000) Markedly increased plasma (1→3)-beta-D-glucan is a diagnostic and therapeutic indicator of Pneumocystis carinii pneumonia in a non-AIDS patient. J Med Microbiol 49(4):393–394
Morris A, Sciurba FC, Norris KA (2011) Pneumocystis: a novel pathogen in chronic obstructive pulmonary disease? COPD 5(1):43–51
Shimizu Y, Sunaga N, Dobashi K et al (2009) Serum markers in interstitial pneumonia with and without Pneumocystis jirovecii colonization: a prospective study. BMC Infect Dis 9:47
Damiani C, Le Gal S, Lejeune D et al (2011) Serum (1- > 3)-beta-D-glucan levels in primary infection and pulmonary colonization with Pneumocystis jirovecii. J Clin Microbiol 49(5):2000–2002
Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A (2013) Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1- > 3)-β-D-glucan for differential diagnosis of Pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol 51(10):3380–3388
Acknowledgments
This research was supported by the scientific projects PTDC/SAU-MII/104231/2008 and PTDC/SAU-MIC/116716/2010 financed by Fundação para a Ciência e a Tecnologia (FCT).
We thank the Associates of Cape Cod, Inc. for providing the free BG detection kit.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esteves, F., Lee, CH., de Sousa, B. et al. (1–3)-Beta-D-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis 33, 1173–1180 (2014). https://doi.org/10.1007/s10096-014-2054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-014-2054-6