Abstract
Sepsis is characterized as an uncontrolled inflammatory response. Spite et al. (Nature 461(7268):1287–1291, 2009) had demonstrated that resolvin D2, which is derived from docosahexaenoic acid (DHA), improves survival in cecal ligation and puncture (CLP)-initiated sepsis and enhances bacterial clearance without immune suppression. Resolvin D1, which is also derived from DHA and homologous with resolvin D2, is an endogenous anti-inflammatory and proresolving lipid molecule. We sought to investigate the effects of resolvin D1 on sepsis and to explore the mechanism of action. Six-to-eight-week-old male C57BL/6 mice were randomly divided into three groups: the sham group underwent the sham operation followed by tail vein injection of vehicle (0.1 % ethanol); the CLP group received vehicle (0.1 % ethanol) after CLP; the resolvin D1 group received resolvin D1 (100 ng) after CLP. Blood, peritoneal lavage fluid, and organs of mice were harvested 24 h after treatment for cytokine analysis, cell counts, bacterial cultures, histopathological studies, and apoptosis quantification. Compared with the vehicle control group, the survival rate and bacterial clearance of mice with sepsis induced by CLP were improved after resolvin D1 treatment, but the numbers of neutrophils in peritoneal lavage fluid, the inflammatory cytokines, the phosphorylation of the nuclear factor-κB (NF-κB) (P65) pathway, and the apoptosis rate of CD3+ T lymphocytes of the thymus were suppressed. Resolvin D1 treatment improved survival in mice with sepsis induced by CLP, enhanced organism bacterial clearance, suppressed the increase of the numbers of neutrophils in peritoneal lavage fluid, reduced the release of inflammatory cytokines, and decreased the apoptosis rate of CD3+ T lymphocytes of the thymus. These results suggest that resolvin D1 may attenuate the degree of inflammatory reaction in sepsis caused by CLP, without harming the host defense response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is defined as the systemic inflammatory response that occurs during severe infection, and it represents an uncontrolled inflammatory response. Sepsis is a huge challenge for the clinician. It requires a large number of medical resources owing to the complex pathogenesis, difficult treatment, and high mortality rates [1, 2].
The pathophysiological process of sepsis is the excessive activation of inflammatory response induced by pathogens and subsequent immune disorder or immunosuppression, which implicates multiple organs, many types of cells, and various pathways. Although our understanding of the mechanisms and the pathogenesis of sepsis have progressed during the last decade, these progresses have not yet yielded the anticipated advantages [3].
More and more studies have shown that lipid metabolism disorder and lipid molecules play an important role in the course of sepsis [4]. There are clinical studies which found that food which is rich in ω-3 polyunsaturated fatty acids [eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)] is beneficial to humans. The consumption of polyunsaturated fatty acids contributes to mitigating inflammatory response and febrile reaction induced by lipopolysaccharides (LPS) in healthy adults. In patients with sepsis, the ratio of ω-3 and ω-6 polyunsaturated fatty acids is an indicator to determine the prognosis of sepsis. It is recommended to use ω3 lipid emulsion to treatment patients with sepsis [5].
Resolvins (resolution-phase interaction products) are a newly identified family of lipid mediators which is generated from the ω-3 fatty acids EPA and DHA during resolution that have potent anti-inflammatory or proresolving actions [6, 7].
Spite et al. [8] found that resolvin D2 improves survival in cecal ligation and puncture (CLP)-initiated sepsis by playing a potent anti-inflammation and proresolving effect in 2009. The meaningful finding demonstrates that the derivants of ω-3 fatty acids may play an important role in the treatment of sepsis.
Resolvin D1 (RvD1) , which is also derived from ω-3 fatty acid DHA, is an important member of the resolvins family [9]. Resolvin D1 can specifically bind in the lipoxin A4 receptor (ALX) and orphan receptor GPR32, block the activity of the nuclear factor-κB (NF-κB), or upregulate proresolving miRNAs [10] to give an anti-inflammatory effect and regulate macrophage phagocytosis through a receptor-dependent mechanism [11] and block excessive leukocytes infiltration into tissues and attenuate the production of proinflammatory cytokines [12]. Resolvin D1 can also reduce inflammatory pain through the central and peripheral mechanisms [13], improve the mouse ischemia-reperfusion injury, and control mouse artery atherosclerosis [14]. In addition, resolvin D1 was found to directly act at a single-cell level in microfluidic chambers to stop human polymorphonuclear neutrophil (PMN) migration to interleukin-8 [15]. Resolvin D1 also protects mice from LPS-induced acute lung injury by reaction with ALX and NF-κB [16, 17] and acute cigarette smoke-induced lung inflammation [18]. Other researchers have found that the endogenous chemical regulators demonstrate unique roles in regulating the absorption of inflammation, inhibiting the infiltration of leukocytes and the production of cytokines, and enhancing the body’s clearance of pathogenic microorganisms [19, 20].
All these findings suggest that resolvin D1 may display potential therapeutic value in sepsis. In this study, we evaluated the regulatory role of resolvin D1 on the immune and inflammatory response in a CLP model of mice.
Materials and methods
Mice
Six-to-eight-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Second Military Medical University (Shanghai, China). Mice were given free access to water and standard rodent chow, and were housed in pathogen-free cages. They were acclimatized for at least one week before use. All experimental procedures involving animals were approved by the Animal Care and Use Committee of Changhai Hospital.
Reagents
Resolvin D1 was purchased from Cayman (CAS Registry No: 872993-05-0, USA). Resolvin D1 was diluted with endotoxin-free saline. Mouse tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Bender MedSystems. Mammalian Protein Extraction Reagent and Protein Assay Kit were purchased from Thermo Scientific.
Cecal ligation and puncture model
CLP was widely used as a model of systemic sepsis syndrome. Healthy male C57BL/6 mice were anesthetized with isoflurane inhalation. A midline abdominal incision was made after the abdomen was disinfected and the cecum was exposed. Then, the cecum was ligated below the ileocecal valve for mid-grade sepsis [21]. A through and through puncture was performed with a 20-gauge needle. The cecum was then placed back into the peritoneal cavity, and the abdominal wall was closed in two layers. As the control, the cecum was exposed but not ligated or punctured, and then returned to the abdominal cavity. All mice were administered 1 ml of sterile saline for fluid resuscitation after recovery from anesthesia.
Experimental protocols
Mice were divided randomly into three groups: (1) sham group: mice underwent the sham operation followed by i.v. administration of vehicle (0.1 % ethanol); (2) CLP group: mice were subjected to CLP followed by i.v. administration of vehicle (0.1 % ethanol); (3) resolvin D1 group: mice were subjected to CLP and treated with resolvin D1 (100 ng) at the time of puncture. At 24 h following operation, blood was collected in a heparinized syringe from the right ventricle, peritoneal lavage fluid was obtained by injecting 2 ml PBS into the peritoneal cavity, and lung, liver, heart, spleen, and thymus tissues were also collected.
Survival analysis
Mice which had undergone CLP were randomized to receive tail vein injection of resolvin D1 or vehicle after CLP (n =16 for each group). The survival of animals was observed for 8 days after operation.
Bacterial cultures
For bacterial culturing, 100 μl peritoneal lavage fluid or blood was diluted with PBS 1:10–1:106, and 100 μl of each dilution was cultured on a tryptic soy blood agar plate. Plates were incubated at 37 °C in aerobic conditions for 24 h, and then the number of colony-forming units (CFU)/ml was counted.
Neutrophils and macrophages/monocytes count in peritoneal lavage fluid and peripheral blood
After erythrocytes were lysed, cells were stained with fluorochrome-conjugated antibody to cell subset-specific surface marker (FITC)-conjugated Gr-1 for neutrophils and (PE)-conjugated F4/80 for macrophages. The stained cells were analyzed using fluorescence-activated cell sorting (FACS) and cell numbers were calculated by flow cytometry (FCM).
Histopathological studies
The animals were sacrificed, and the heart, lung, and liver were immediately removed. The specimens were fixed in 4 % paraformaldehyde for 12–24 h, embedded in paraffin, sectioned into 5-μm slices, and stained with hematoxylin and eosin (H&E). Histological changes were examined by a pathologist who was blinded to the identification of the treatment groups and scored according to the pathologic scoring system as previously described [22].
Quantification of apoptosis in the thymus
Thymus tissues were harvested from mice 24 h after surgery (n = 6 for each group), and single-cell suspensions were prepared. The apoptotic rate of CD3+ T cells was detected using an annexin V-FITC binding and propidium iodide (PI) staining apoptosis detection kit (R and D Systems, Abingdon, UK), as described in the manufacturer’s instructions. Stained cells were analyzed via the FACSCalibur apparatus and the CellQuest software (Becton Dickinson, USA). T cells positive for CD3 were first analyzed and cells positive for annexin V in CD3+ T cells were determined to be apoptotic cells.
Cytokine assays
Levels of TNF-α, IL-6, IL-10, and IL-17 in plasma were measured using the murine ELISA kit (Bender Systems, USA), according to the manufacturer’s instructions.
Western blotting
The lungs of the mice were lysed by Mammalian Protein Extraction Reagent. The concentrations of the proteins were determined by the BCA protein assay kit. Protein extracts were fractionated on 10 % sodium dodecyl sulfate-polyacrylamide (SDS) gel and then transferred to the PVDF membrane. The membrane was blocked with 5 % fat-free milk in Tris-buffered saline (TBS) containing 0.1 % Tween-20, followed by incubation with rabbit primary polyclonal antibody of phosphorylated/total NF-κB at 4°C overnight. Then, the membrane was treated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:3,000). Antibody binding was visualized with an ECL chemiluminescence system. The intensity was quantified by using Imager software (UVP, USA).
Statistical analysis
Data are reported as the mean ± standard error of the mean (SEM). All statistical analyses were performed using Prism 5.0 (GraphPad Software, USA).
Survival of the two subgroups was estimated by Kaplan–Meier survival curves; comparisons were performed by the log-rank test. All comparisons among groups were performed by one-way analysis of variance (ANOVA). For multigroup analysis, intergroup comparisons were performed by the Newman–Keuls multiple comparison test. A significance level of 0.05 was considered to be significant for all calculations.
Result
RvD1 improves survival in mice with sepsis induced by CLP
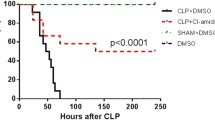
In order to investigate whether resolvin D1 was of benefit to CLP mice, survival was observed for 8 days after CLP. Sepsis resulted in a mortality rate of 81.25 % within 8 days, which is much lower than that in the sham group (100 %; p < 0.05). In contrast, animals treated with resolvin D1 (100 ng, i.v.) after CLP showed a significantly improved survival rate during the study period (37.5 %; p < 0.05) (Fig. 1).
Survival rate of septic mice. Resolvin D1 improves the survival of polymicrobial sepsis in cecal ligation and puncture (CLP) mice. In the resolvin D1 group, mice were treated with intravenous resolvin D1 (100 ng/mice) immediately after CLP. An equal amount of saline was administered in the sham and CLP groups. Survival analyses were carried out with the log-rank test. *p < 0.05
RvD1 enhances bacterial clearance in the CLP model
Blood and peritoneal lavage fluid samples were harvested 24 h after CLP and the CFU was determined. Decreased bacterial burden was found in the blood and peritoneal lavage fluid samples in the mice treated with resolvin D1 after CLP compared with the CLP group. These data indicate that resolvin D1 enhanced the clearance of bacterial burden from the blood and peritoneal lavage fluid of sepsis mice (Fig. 2).
Bacterial clearance in blood (a) and peritoneum (b) in mice. Resolvin D1 enhanced bacterial clearance in blood (a) and peritoneum (b). Blood and peritoneal lavage fluid were harvested 24 h after CLP. Data analyses were carried out with one-way analysis of variance (ANOVA) and the Newman–Keuls multiple comparison test. *p < 0.05, **p < 0.01
Changes of pathological scores for severity of lung injury
Lungs in CLP mice were characterized by a large accumulation of inflammatory cells and exudates infiltration in lumina and alveolars, capillary distended, alveolar wall widened, or consolidation, while the administration of resolvin D1 resulted in significant attenuation of these findings (Fig. 3).
Histopathological changes. The lung tissues were harvested 24 h after CLP for histopathologic examination using hematoxylin and eosin (H&E) staining. There were six animals in each group and representative images are shown. Histopathological findings showed milder impairment in the lungs after resolvin D1 administration. a After resolvin D1 treatment, the lungs showed less congestion in the alveolar wall and a widened alveolar wall. b The severity of lung injury was scored as described in the Materials and methods section. # p < 0.05 vs. sham, *p < 0.05 vs. CLP. RvD1 resolvin D1 group; CLP CLP group
Effect of resolvin D1 on the numbers of neutrophils in the blood and peritoneal lavage fluid of CLP mice
We detected the amounts of neutrophils in the blood and peritoneal lavage fluid of CLP mice (Fig. 4).The findings showed that treatment with resolvin D1 suppressed increase in the numbers of neutrophils in peritoneal lavage fluid compared with the CLP group, whereas the numbers of neutrophils in the blood of the resolvin D1 group were not significantly different to that of the CLP group.
The number of neutrophils in the peritoneal cavity and blood. Peritoneal lavage fluid and blood were harvested 24 h after CLP. a Resolvin D1 reduced the number of neutrophils in peritoneum. b The number of neutrophils in blood did not show significant differences. RvD1 resolvin D1 group; CLP CLP group. Data analyses were carried out with one-way ANOVA and the Newman–Keuls multiple comparison test. **p < 0.01
Resolvin D1 reduced the release of inflammatory cytokines
At 24 h after operation, the levels of some inflammatory cytokines were detected in plasma. The levels of IL-1β, TNF-α, IFN-γ, and IL-10 were significant lower in the resolvin D1 treatment group than that of the CLP group (Fig. 5).
Cytokines expression in the blood of mice. Samples were collected 24 h after CLP. Resolvin D1 reduced the production of proinflammatory cytokines and anti-inflammatory cytokine. Proinflammatory cytokines included TNF-α, IL-6, and IFN-γ. IL-10 represented the anti-inflammatory cytokine. RvD1 resolvin D1 group; CLP CLP group. Data analyses were carried out with one-way ANOVA and the Newman–Keuls multiple comparison test. *p < 0.05, **p < 0.01
T lymphocytes apoptosis in the thymus and cell counts in the spleen
Sepsis-induced immunosuppression is characterized by increased apoptosis of lymphocytes and, hence, reduced lymphocyte counts. Resolvin D1 significantly decreased the apoptosis rate of CD3+ T lymphocytes of the thymus in the CLP model. However, the numbers of CD4+ T lymphocytes and macrophages in the spleen of the CLP group were similar to that of the resolvin D1 group (Fig. 6).
Apoptosis in the thymus and cell counts in the spleen. Resolvin D1 inhibited lymphocyte apoptosis in the thymus. The numbers of CD4+ T lymphocytes and macrophages in the spleen of the CLP and resolvin D1 groups were significantly lower than that of sham group, but there were no significant differences between the CLP and resolvin D1 groups. RvD1 resolvin D1 group; CLP CLP group. Data analyses were carried out with one-way ANOVA and the Newman–Keuls multiple comparison test. *p < 0.05
Effects of resolvin D1 on the NF-κB signaling pathway in the lung tissues of sepsis mice
Activation of the NF-κB signaling pathway plays a key role in regulating the production of inflammatory mediators. Therefore, we further examined the activation of NF-κB(P65). As shown in Fig. 7, the phosphorylation of NF-κB(P65) was increased in the lung tissues of the sepsis group compared with the sham group, as determined by Western blotting analysis with phosphorylated antibodies of NF-κB(P65). The injection of resolvin D1 (10 ng, 100 ng) via the tail vein to the mice could inhibit the phosphorylation of NF-κB(P65) dose-dependently.
Discussion
Sepsis is defined as the systemic inflammatory response which occurs during severe infection. It is characterized by an uncontrolled inflammatory response and subsequent immunosuppression. Sepsis and sepsis-associated multiorgan failure has been a major challenge for both scientists and clinicians [23]. The model of CLP is an established murine polymicrobial sepsis that closely resembles humans and is currently considered as the gold standard in sepsis research [24–26]. Landmark research was carried out by Spite et al. [8], who found that resolvin D2, which is derived from DHA, improves survival in CLP-initiated sepsis and enhances bacterial clearance without immune suppression. The finding indicated that resolvins may play an important role in the treatment of sepsis. Resolvin D1 is also derived from DHA and plays a similar role in the absorption of inflammation. But because they act on different receptors, whether or not resolvin D1 has an influence on the treatment of sepsis similar to resolvin D2 requires further study.
This study is designed to evaluate the effect of resolvin D1 on microbial sepsis initiated by CLP in mice. Our research data indicate that treating sepsis with resolvin D1 improves the survival rate of CLP mice and shows beneficial treatment effects, including enhanced bacterial clearance of blood and peritoneal lavage fluid, attenuated excessive inflammatory response, and inhibited uncontrolled neutrophil migration. In addition, the apoptosis rate of T cells in the thymus was markedly reduced following resolvin D1 treatment, and the phosphorylation of NF-κB(P65) could be inhibited dose-dependently.
There are many researchers who found that resolvin D1 has potent anti-inflammatory, pro-resolving, and analgesic actions. For example, resolvin D1 demonstrated organ-protective actions in murine ischemia reperfusion injury and acts directly on single human PMN to regulate tissue inflammation [15]. Resolvin D1 interfered with the transendothelial migration of human neutrophils [7], suppressed IL-1β transcription induced by TNF-α in microglia, and reduced polymorphonuclear leukocyte infiltration [9], protected mice from LPS-induced acute lung injury by selective reaction with ALX, which inhibits MAPKs and the NF-κB pathway [27], and very low doses (1 μg/kg) of aspirin-triggered resolvin D1 (AT-RvD1) can reduce inflammatory pain in an adjuvant-induced arthritis model [28].
In our study, we found some phenomena that are consistent with some of the published literature, such as tail vein injection of resolvin D1 suppressed the increase in the numbers of neutrophils in peritoneal lavage fluid [29] and reduced the release of inflammatory cytokines. These effects may be helpful to increase the survival rate and prevent tissue damage. However, the numbers of neutrophils in the blood did not show a significant reduction. We suggest that it may be related to resolvin D1 limiting the transendothelial migration of neutrophils [17].
In addition, resolvin D1 enhanced the clearance of bacterial burden from the blood and peritoneal lavage fluid of sepsis mice. There is evidence that resolvin D1 enhanced the macrophage phagocytosis of zymosan and apoptotic PMNs by specific binding on human ALX and GPR32 [11, 14]. So resolvin D1 may enhance bacterial clearance by the receptor-dependent pathway on the macrophages of mice.
The extensive apoptotic of lymphocytes is likely an important cause of the immunosuppression which is a hallmark of patients with sepsis. The potential importance of apoptosis in the pathophysiology of sepsis is illustrated by the results from animal models which demonstrate that blocking lymphocytes apoptosis improves survival in sepsis [30]. In our study, we found that the apoptosis rate of CD3+ T lymphocytes of thymus was reduced after administration with resolvin D1. There are researchers who found that resolvin E1 (generated from the ω-3 fatty acid EPA) can enhance phagocytosis-induced neutrophil apoptosis and mitigate prosurvival signals [31], the effect of which may be related to neutrophil phagocytosis evoking a rapid, robust ROS production and increased caspase-8 and caspase-3 activity. But as far as the authors know, there is no evidence to indicate that resolvins can affect the apoptosis of lymphocytes by the receptor-dependent pathway or directly act on caspase 3. However, some researchers have reported improved clinical findings by decreasing circulating cytokines in septic patients undergoing hemopurification [32]. The basis of the theory of hemofiltration is to attenuate the inflammatory response by removing the very high peaks in proinflammatory cytokines that are produced. Moreover, uncontrolled cytokines might trigger apoptosis. So, resolvin D1 may inhibit lymphocyte apoptosis by attenuating uncontrolled inflammatory responses.
The major limitation of this study is that we have not progressively explored the specific mechanism underlying the anti-inflammatory and anti-apoptotic effects of resolvin D1. Further studies should aim to uncover the mechanism underlying the anti-inflammatory and anti-apoptotic effects of resolvin D1, as well as to examine the efficacy of resolvin D1 in patients in the clinical setting.
It is well known that it was the host response, rather than that of the microorganism, which is the most responsible for the morbidity and mortality associated with the disorder. Resolvin D1 is a potent lipid regulator with anti-inflammatory effects, activating inflammation-resolution programs and enhancing host defense. All of the beneficial effects of resolvin D1 demonstrate that it may be an important potential therapeutic measure against sepsis [33].
Abbreviations
- RvD1:
-
Resolvin D1
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- CLP:
-
Cecal ligation and puncture
- PMN:
-
Polymorphonuclear neutrophil
References
Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Kumar V, Sharma A (2008) Innate immunity in sepsis pathogenesis and its modulation: new immunomodulatory targets revealed. J Chemother 20:672–683
Chiu WC, Wang YC, Chien YW, Hou YC, Hu YM, Yeh SL (2009) Effects of dietary fish oil supplementation on cellular adhesion molecule expression and tissue myeloperoxidase activity in hypercholesterolemic mice with sepsis. J Nutr Biochem 20(4):254–260
Krogh-Madsen R, Plomgaard P, Akerstrom T, Møller K, Schmitz O, Pedersen BK (2008) Effect of short-term intralipid infusion on the immune response during low-dose endotoxemia in humans. Am J Physiol Endocrinol Metab 294:E371–E379
Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chioléro RL (2007) Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive Care Med 33:789–797
Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL (2002) Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196:1025–1037
Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461(7268):1287–1291
Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN (2003) Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278:14677–14687
Krishnamoorthy S, Recchiuti A, Chiang N, Fredman G, Serhan CN (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am J Pathol 180(5):2018–2027
Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN (2010) Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A 107:1660–1665
Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD (2012) Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol 189(4):1983–1991
Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR (2010) Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med 16(5):592–597, 1p following 597
Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L (2008) Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J 22:3595–3606
Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN (2008) Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol 181(12):8677–8687
Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F (2012) Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respir Res 13:110
Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD (2013) Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 6(2):256–266
Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ (2013) A novel anti-inflammatory and pro-resolving role for resolvin d1 in acute cigarette smoke-induced lung inflammation. PLoS One 8(3):e58258
Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M (2011) Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese–diabetic mice. FASEB J 25(7):2399–2407
Schwab JM, Chiang N, Arita M, Serhan CN (2007) Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 447(7146):869–874
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4:31–36
Hou LC, Qin MZ, Zheng LN, Lu Y, Wang Q, Peng DR, Yu XP, Xin YC, Ji GL, Xiong LZ (2009) Severity of sepsis is correlated with the elevation of serum high-mobility group box 1 in rats. Chin Med J (Engl) 122(4):449–454
Dombrovskiy VY, Martin AA, Sunderram J, Paz HL (2007) Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 35:1244–1250
Rittirsch D, Hoesel LM, Ward PA (2007) The disconnect between animal models of sepsis and human sepsis. J Leukoc Biol 81:137–143
Remick DG, Newcomb DE, Bolgos GL, Call DR (2000) Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock 13:110–116
Deitch EA (2005) Rodent models of intra-abdominal infection. Shock 24(Suppl 1):19–23
Wang B, Gong X, Wan JY, Zhang L, Zhang Z, Li HZ, Min S (2011) Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther 24(4):434–441
Xu ZZ, Ji RR (2011) Resolvins are potent analgesics for arthritic pain. Br J Pharmacol 164(2):274–277
Norling LV, Dalli J, Flower RJ, Serhan CN, Perretti M (2012) Resolvin D1 limits polymorphonuclear leukocyte recruitment to inflammatory loci: receptor-dependent actions. Arterioscler Thromb Vasc Biol 32(8):1970–1978
Hotchkiss RS, Coopersmith CM, Karl IE (2005) Prevention of lymphocyte apoptosis—a potential treatment of sepsis? Clin Infect Dis 41(Suppl 7):S465–S469
El Kebir D, Gjorstrup P, Filep JG (2012) Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A 109(37):14983–14988
Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS (2010) IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol 184(3):1401–1409
Chiang N, Fredman G, Bäckhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484(7395):524–528
Acknowledgment
We thank professor JinBao Li for the design of the experiment and the valuable discussion.
Conflict of interest
The authors declare that they have no competing interests.
Feng Chen, YouPing Wu, and JiaLi Zhu carried out the CLP model and participated in the immunoassays. Feng Chen, Fei Wang, and LuLong Bo carried out the flow cytometry calculations and statistical analysis. Feng Chen, XiaoHua Fan, Rui Bao, and XiaoMing Deng conceived the study and participated in the design and coordination and drafted the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Feng Chen and XiaoHua Fan contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Chen, F., Fan, X.H., Wu, Y.P. et al. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur J Clin Microbiol Infect Dis 33, 457–464 (2014). https://doi.org/10.1007/s10096-013-1978-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1978-6