Abstract
Yersinia enterocolitica biotype 1A strains are frequently isolated from the environment, foods, and animals, and also from humans with yersiniosis. There are controversial reports on the pathogenicity of biotype 1A strains. In this study, 811 fecal samples from asymptomatic humans from Switzerland were studied for the presence of Y. enterocolitica. Nine (1.1 %) of the 811 samples were positive for Y. enterocolitica 1A. These strains were compared with 12 Y. enterocolitica 1A strains from Swiss patients with diarrhea isolated in the same year. Almost all (20/21) Y. enterocolitica 1A strains carried the ystB gene, seven strains carried the hreP gene, and none carried the ail, ystA, myfA, yadA, or virF genes. Most (17/21) Y. enterocolitica 1A strains belonged to two major clusters, A and B, by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Strains of cluster B were only isolated from humans with diarrhea; however, ystB and hreP genes were detected in strains from both clinical and non-clinical samples and from strains of clusters A and B. Using ribotyping, six restriction patterns among biotype 1A strains were obtained with HindIII enzyme. The most common ribotype (RT I) was found in strains isolated from humans with and without diarrhea. All biotype 1A strains had a unique NotI profile by pulsed-field gel electrophoresis (PFGE), showing a very high genetic diversity. In this study, Y. enterocolitica 1A strains from clinical and non-clinical samples could not be clearly differentiated from each other. More research is needed in order to prove that biotype 1A strains are a primary cause for human yersiniosis and not only a secondary finding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yersinia enterocolitica is an important enteric bacterium causing gastrointestinal problems, long-term sequelae like reactive arthritis, and, sometimes, septicemia due to blood transfusion [4, 9]. Y. enterocolitica represents six biotypes (1A, 1B, 2–5) [2]. Strains belonging to biotypes 1B and 2–5 carry the virulence plasmid (pYV) and the chromosomal genes ail, ystA, myfA, and hreP, and are, thus, considered pathogenic to humans and animals. Strains belonging to biotype 1A are considered non-pathogenic because they do not carry the pYV and the important chromosomal virulence genes are missing [3, 5].
Biotype 1A strains are widely distributed in the environment and have frequently been isolated from samples of food and animal origin [2]. However, strains of biotype 1A have also been isolated from symptomatic humans [6, 18, 20]. In Finland and Switzerland, biotype 1A strains are common findings in the feces of diarrheic humans [6, 20]. Still, septicemia and reactive arthritis have only rarely been reported [2].
The accurate identification of Y. enterocolitica can be difficult if only biochemical tests are used [20]. Especially, Y. massiliensis, Y. mollaretii, Y. bercovieri, and Y. rohdei are very easily misdiagnosed as Y. enterocolitica with phenotypic methods. Information of the biotype and virulence genes is needed for a proper assessment of the potential pathogenicity of the Yersinia strain [20]. Y. enterocolitica 1A strains are serologically very heterogeneous [2] and they show clearly wider genetic diversity than the human pathogenic strains belonging to biotypes 2 and 4 [6, 15]. Y. enterocolitica 1A strains have been reported to sometimes carry chromosomal virulence genes like myfA, ystB, and hreP [3].
There are controversial reports on the pathogenicity of clinical and non-clinical Y. enterocolitica biotype 1A strains and, thus, Yersinia strains from humans with and without diarrhea were collected in Switzerland during 2011 for further characterization using phenotypic and genotypic methods.
Materials
In total, 811 fecal samples from asymptomatic humans collected in 2011 in Switzerland were studied for the presence of Yersinia spp. The samples were from humans between the ages of 20 and 60 years. Most (641/811) of the samples were from males, 166 samples were from females, and for four samples, the gender was not known. Furthermore, 26 Yersinia spp. strains isolated in 2011 from patients with diarrhea in Switzerland were characterized and compared with the strains isolated from asymptomatic humans of the same year (Table 1).
Methods
About a 1-g fecal sample was mixed in 9 ml PMB (peptone broth supplemented with 1 % mannitol and 0.15 % bile salts) [16]. Cold enrichment at 4 °C for 3 weeks was used for all samples before plating on Yersinia-selective CIN (cefsulodin–irgasan–novobiocin) agar (Oxoid AG, Basel, Switzerland). The CIN plates were incubated at 30 °C for 24 to 48 h. Presumptive positive colonies were subcultured on blood agar and then tested for the urease enzyme. Urease-positive colonies were identified with API 20E and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) [6, 22].
Y. enterocolitica isolates were bio- and serotyped [6]. The biotype was determined using pyrazinamidase and Tween activity, esculin hydrolysis, indole production, and salicin, xylose, and trehalose fermentation, and serotyping was carried out with slide agglutination using commercial Y. enterocolitica O:1-O:3, O:5, O:9 (Denka Seiken, Tokyo, Japan), and O:27 antisera (Sifin, Berlin, Germany).
Seven genes were studied by polymerase chain reaction (PCR): two virulence genes (yadA and virF) located on the pYV of the pathogenic Yersinia spp. and five chromosomal virulence genes (ail, ystA, ystB, myfA, and hreP) [3, 12, 23, 24]. The DNA was released from bacterial colonies by heating at 99 °C for 10 min, and 1 μl of this liquid was added to 19 μl of the master mix, which contained 1× ready-to-use mix (iQ™ SYBR Green Supermix, Bio-Rad, Hercules, CA) and 200 nM of primers. All genes were studied separately in a single PCR. The fluorescence intensity of the SYBR Green Supermix and the melting curve analysis were studied using the CFX96 system (Bio-Rad). A threshold cycle (Ct) under 30 and a specific melting temperature (Tm) indicated a positive result.
Moreover, the Yersinia strains (one isolate per sample) were genotyped. The 16S and 23S restriction fragment length polymorphism (ribotyping) of the strains was studied using HindIII restriction enzymes [7] and NotI enzyme was used for pulsed-field gel electrophoresis (PFGE) [8]. The Dice correlation coefficient and unweighted pair-group method with arithmetic mean (UPGMA) clustering were used for constructing the dendrogram (Fig. 1).
Results
Nine (1.1 %) of the 811 fecal samples from asymptomatic humans were Y. enterocolitica biotype 1A-positive. Y. enterocolitica was the only Yersinia species isolated from the samples and biotype 1A was the only biotype identified.
In total, 26 clinical Y. enterocolitica strains were sent to the Yersinia reference laboratory in Switzerland during the year 2011. Fourteen (54 %) of the 26 strains belonged to biotypes 2 or 4 and 12 (46 %) to biotype 1A (Table 1). Seven (50 %) of the 14 strains of biotypes 2 or 4 but only one (8 %) of the 12 biotype 1A strains were isolated from humans under 20 years old.
Biotype 1A strains have frequently been isolated in the fecal samples of humans with diarrhea in Switzerland (Table 2). In 2011, biotype 1A was the most common type (46 %) found in the fecal samples of humans with diarrhea, followed by biotypes 4 (35 %) and 2 (19 %).
Most (86 %) of the 14 Y. enterocolitica strains belonging to biotypes 2 or 4 were identified with a high ID% using API 20E (Table 3). Only two of these strains (14 %) had a low or no ID% (https://apiweb.biomerieux.com). Five (24 %) of the 21 Y. enterocolitica biotype 1A strains could not be identified with a high ID% using API 20E. One of these strains was even identified as Serratia marcescens. Using MALDI-TOF MS, all Y. enterocolitica biotype 1A strains were identified as Y. enterocolitica and were distinguished from Y. enterocolitica biotypes 2 and 4 strains.
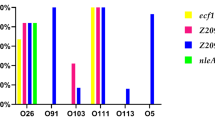
All 14 Y. enterocolitica strains of biotypes 2 or 4 carried the ail, ystA, myfA, and hreP genes in the chromosome, but were ystB-negative (Table 4). Most (79 %) of the biotypes 2 and 4 strains also carried yadA and virP genes on the pYV. Almost all (95 %) Y. enterocolitica 1A strains carried ystB in the chromosome, but only one strain was negative. The ribotype pattern (RT VI) of the ystB-negative strain differed clearly from the ribotype patterns (RT I–V) of the ystB-positive strains (Fig. 1). The hreP was detected in 7 (33 %) Y. enterocolitica 1A strains (Table 5). None of the Y. enterocolitica 1A strains carried the yadA or virP genes.
Most (81 %) Y. enterocolitica 1A strains belonged to two major clusters, A and B, by MALDI-TOF MS. All but one strain from humans without diarrhea belonged to cluster A and strains of cluster B were only isolated from clinical stool samples (Table 5). Strains carrying ystB and hreP were found in clinical and non-clinical strains and in strains from both A and B clusters. Using ribotyping, six different restriction patterns (ribotypes), RT I–VI, were obtained with HindIII enzyme (Fig. 1). Ten (48 %) of the Y. enterocolitica 1A strains expressed ribotype I and they were isolated from humans with and without diarrhea (Table 5). Ribotype II was found in three strains, which were all from clinical samples. Different NotI profiles were obtained in all biotype 1A strains and no clear clustering between the strains according source or any other identifiable determinant could be seen.
Discussion
The prevalence of Y. enterocolitica 1A in fecal samples from asymptomatic humans was only 1.1 %, which is surprisingly low, since Y. enterocolitica 1A and other non-pathogenic Yersinia spp. are frequently isolated from different food samples [2].
During 2011, 26 Y. enterocolitica strains from humans with diarrhea were sent to the national reference laboratory for Yersinia. Three biotypes (1A, 2, and 4) were identified. The most common biotype was 1A, which has frequently been isolated from the clinical fecal samples of symptomatic patients in Switzerland during the last decade [6]. Direct culturing is mostly used for clinical samples in Switzerland, which indicates that the number of Y. enterocolitica 1A is high in fecal samples from humans with diarrhea. Biotype 1A strains have also been reported to be common in Finnish patients. However, in Finland, cold enrichment, which supports the growth of all psychrotrophic Yersinia spp., is used also for clinical samples [20].
Y. enterocolitica strains of biotypes 2 or 4 were only isolated from the fecal samples of humans with diarrhea, which shows that asymptomatic humans do not usually shed strains of these biotypes. Strains of biotypes 2 and 4 were frequently isolated from young patients (under 20 years of age). Y. enterocolitica 1A strains were isolated from fecal samples from both symptomatic and asymptomatic humans. In Finland, the symptoms and sources of patients with Y. enterocolitica 1A strains differed from those patients with strains of biotypes 2 and 4. The patients with biotype 1A strains were adults with more long-lasting, unspecific symptoms, which suggests that the original cause of illness may have been something other than Y. enterocolitica 1A [13].
The identification of Y. enterocolitica strains by phenotypic methods has been shown to be very laborious and accurate identification is difficult [20]. However, the authors reported that, by combining API 20E and biotyping, Y. enterocolitica strains belonging to biotypes 2–5 can be identified reliably. In this study, Yersinia strains were identified with API 20E and MALDI-TOF MS. Some discrepancies in the identification of Y. enterocolitica occurred if only API 20E was used. All Y. enterocolitica 1A strains were differentiated from Y. enterocolitica biotypes 2 and 4 strains by MALDI-TOF MS, which is a convenient method to identify a high number of bacterial strains rapidly [22].
Distribution of the virulence genes differed between the biotype 1A strains and biotypes 2 and 4 strains. All Y. enterocolitica strains of biotypes 2 or 4 carried the chromosomal ail, ystA, myfA, and hreP genes. Furthermore, virP and yadA located on the pYV were detected in most of the biotypes 2 and 4 strains. All Y. enterocolitica 1A strains were virF, yadA, ail, ystA, and myfA negative. The pYV has, so far, not been found in Y. enterocolitica 1A strains and, thus, virP and yadA have also not been detected in biotype 1A strains. In our earlier study, one of the 51 human clinical Y. enterocolitica 1A strains carried the ail gene in Switzerland [6]. Recently, the ail gene was also detected in some biotype 1A strains in Germany and Finland [14, 19]. However, ail and ystA have very seldom been detected among biotype 1A strains [3]. All but one Y. enterocolitica 1A strain carried the ystB gene. It has been demonstrated that Y. enterocolitica can produce heat-stable enterotoxins. YstB is usually produced by strains belonging to biotype 1A and the enterotoxin YstA by strains belonging to biotypes 1B and 2–5. Singh and Virdi [21] showed that the ystB gene is widely distributed among human clinical isolates and that the production of YstB enterotoxin can be induced at the conditions found in ileum (37 °C, pH 7.5), indicating that YstB is an important virulence determinant in biotype 1A strains [21]. However, in this study, ystB was also detected in all Y. enterocolitica 1A strains isolated from asymptomatic humans. Furthermore, the hreP gene was detected among clinical and non-clinical strains. The strain carrying the ystB and hreP genes may have some pathogenic potential, but more research is needed. The myfA gene has been shown to be more predominant in Indian biotype 1A strains than in European strains. Batzilla et al. [1] sequenced two Y. enterocolitica 1A strains and found, in both strains, ystB, myfA, and hreP, but not ail and ystA genes. However, myfA in both biotype 1A strains showed sequence variability and differed from highly conserved myfA in biotypes 1B and 4 strains [1]. This sequence variability may explain the failure to detect the myfA gene in biotype 1A strains.
Two major clonal groups of Y. enterocolitica 1A have been reported earlier by different genotypic and phenotypic methods [10, 11, 17, 22]. Furthermore, Bhagat and Virdi have shown a correlation between the distribution of virulence-associated genes myfA, ystB, and hreP and the clonal groups [3]. In this study, Y. enterocolitica 1A strains were grouped into two major clusters using MALDI-TOF MS; most of the non-clinical strains were grouped into cluster A and most of the clinical strains were grouped into cluster B. However, no correlation between the distribution of the virulence-associated genes and the clonal groups was seen; ystB and hreP were distributed in strains of both clonal groups. In the earlier study by Bhagat and Virdi [3], the distribution of virulence-associated genes between clinical and non-clinical strains did not significantly differ, which is in accordance with our results. Furthermore, no clear clustering of non-clinical and clinical strains was obtained by ribotyping and PFGE. All 1A strains revealed different NotI profiles by PFGE, showing a high genetic diversity among these strains. These results could not show any clear difference between Y. enterocolitica 1A strains isolated from humans with diarrhea and without diarrhea. More research is needed in order to prove the significance of biotype 1A strains in human yersiniosis.
References
Batzilla J, Heesemann J, Rakin A (2011) The pathogenic potential of Yersinia enterocolitica 1A. Int J Med Microbiol 301:556–561
Bhagat N, Virdi JS (2011) The enigma of Yersinia enterocolitica biovar 1A. Crit Rev Microbiol 37:25–39
Bhagat N, Virdi JS (2007) Distribution of virulence-associated genes in Yersinia enterocolitica biovar 1A correlates with clonal groups and not the source of isolation. FEMS Microbiol Lett 266:177–183
Cover TL, Aber RC (1989) Yersinia enterocolitica. N Engl J Med 321:16–24
Falcão JP, Falcão DP, Pitondo-Silva A, Malaspina AC, Brocchi M (2006) Molecular typing and virulence markers of Yersinia enterocolitica strains from human, animal and food origins isolated between 1968 and 2000 in Brazil. J Med Microbiol 55:1539–1548
Fredriksson-Ahomaa M, Cernela N, Hächler H, Stephan R (2012) Yersinia enterocolitica strains associated with human infections in Switzerland 2001–2010. Eur J Clin Microbiol Infect Dis 31:1543–1550
Fredriksson-Ahomaa M, Murros-Kontiainen A, Säde E, Puolanne E, Björkroth J (2012) High number of Yersinia enterocolitica 4/O:3 in cold-stored modified atmosphere-packed pig cheek meat. Int J Food Microbiol 155:69–72
Fredriksson-Ahomaa M, Hallanvuo S, Korte T, Siitonen A, Korkeala H (2001) Correspondence of genotypes of sporadic Yersinia enterocolitica bioserotype 4/O:3 strains from human and porcine sources. Epidemiol Infect 127:37–47
Guinet F, Carniel E, Leclercq A (2011) Transfusion-transmitted Yersinia enterocolitica sepsis. Clin Infect Dis 53:583–591
Gulati P, Varshney RK, Virdi JS (2009) Multilocus variable number tandem repeat analysis as a tool to discern genetic relationships among strains of Yersinia enterocolitica biovar 1A. J Appl Microbiol 107:875–884
Gulati PS, Virdi JS (2007) The rrn locus and gyrB genotyping confirm the existence of two clonal groups in strains of Yersinia enterocolitica subspecies palearctica biovar 1A. Res Microbiol 158:236–243
Heusipp G, Young GM, Miller VL (2001) HreP, an in vivo-expressed protease of Yersinia enterocolitica, is a new member of the family of subtilisin/kexin-like proteases. J Bacteriol 183:3556–3563
Huovinen E, Sihvonen LM, Virtanen MJ, Haukka K, Siitonen A, Kuusi M (2010) Symptoms and sources of Yersinia enterocolitica-infection: a case–control study. BMC Infect Dis 10:122
Kraushaar B, Dieckmann R, Wittwer M, Knabner D, Konietzny A, Mäde D, Strauch E (2011) Characterization of a Yersinia enterocolitica biotype 1A strain harbouring an ail gene. J Appl Microbiol 111:997–1005
Boghenbor KK, On SLW, Kokotovic B, Baumgartner A, Wassenaar TM, Wittwer M, Bissig-Choisat B, Frey J (2006) Genotyping of human and porcine Yersinia enterocolitica, Yersinia intermedia, and Yersinia bercovieri strains from Switzerland by amplified fragment length polymorphism analysis. Appl Environ Microbiol 72:4061–4066
Laukkanen R, Hakkinen M, Lundén J, Fredriksson-Ahomaa M, Johansson T, Korkeala H (2010) Evaluation of isolation methods for pathogenic Yersinia enterocolitica from pig intestinal content. J Appl Microbiol 108:956–964
Mallik S, Virdi JS (2010) Whole cell protein profiling reiterate phylogenetic relationships among strains of Yersinia enterocolitica biovar 1A as discerned earlier by different genotyping methods. J Appl Microbiol 109:946–952
McNally A, Cheasty T, Fearnley C, Dalziel RW, Paiba GA, Manning G, Newell DG (2004) Comparison of the biotypes of Yersinia enterocolitica isolated from pigs, cattle and sheep at slaughter and from humans with yersiniosis in Great Britain during 1999–2000. Lett Appl Microbiol 39:103–108
Sihvonen LM, Hallanvuo S, Haukka K, Skurnik M, Siitonen A (2011) The ail gene is present in some Yersinia enterocolitica biotype 1A strains. Foodborne Pathog Dis 8:455–457
Sihvonen LM, Haukka K, Kuusi M, Virtanen MJ, Siitonen A; YE study group (2009) Yersinia enterocolitica and Y. enterocolitica-like species in clinical stool specimens of humans: Identification and prevalence of bio/serotypes in Finland. Eur J Clin Microbiol Infect Dis 28:757–765
Singh I, Virdi JS (2004) Production of Yersinia stable toxin (YST) and distribution of yst genes in biotype 1A strains of Yersinia enterocolitica. J Med Microbiol 53:1065–1068
Stephan R, Cernela N, Ziegler D, Pflüger V, Tonolla M, Ravasi D, Fredriksson-Ahomaa M, Hächler H (2011) Rapid species specific identification and subtyping of Yersinia enterocolitica by MALDI-TOF mass spectrometry. J Microbiol Methods 87:150–153
Thisted Lambertz S, Nilsson C, Hallanvuo S, Lindblad M (2008) Real-time PCR method for detection of pathogenic Yersinia enterocolitica in food. Appl Environ Microbiol 74:6060–6067
Weynants V, Jadot V, Denoel PA, Tibor A, Letesson JJ (1996) Detection of Yersinia enterocolitica serogroup O:3 by a PCR method. J Clin Microbiol 34:1224–1227
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stephan, R., Joutsen, S., Hofer, E. et al. Characteristics of Yersinia enterocolitica biotype 1A strains isolated from patients and asymptomatic carriers. Eur J Clin Microbiol Infect Dis 32, 869–875 (2013). https://doi.org/10.1007/s10096-013-1820-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-013-1820-1