Abstract
Despite recommendations, gastric aspirate collected by invasive nasogastric aspiration is still routinely used for the direct detection of Mycobacterium tuberculosis in our institution. Reviewing 82 patients with culture-proven respiratory tuberculosis over a 28-month period, we observed no patient diagnosed solely by gastric aspirate analysis. Moreover, the diagnosis yield of gastric aspirate (60 %) did not significantly differ from that of stool specimen (64 %). These data confirm that gastric aspirate is no longer useful for the diagnosis of respiratory tuberculosis contrary to stool specimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary tuberculosis remains one of the most widespread bacterial infections, with an estimated 8.8 million new cases worldwide in 2010 [1]. Because pulmonary tuberculosis remains a deadly communicable infection, its diagnosis remains of great importance to the management of patients and contacts. Despite extensive studies aimed to develop indirect diagnosis assays, such as interferon-releasing assays, the diagnosis of pulmonary tuberculosis still relies on direct diagnosis based on the molecular detection of Mycobacterium tuberculosis-specific sequences and the isolation and culture of M. tuberculosis organisms [2]. The latter approach allows for further characterization of the isolate, including genotyping [3] and antibiotic susceptibility testing, which are of great importance due to the emergence of antibiotic-resistant M. tuberculosis strains [4].
Although gastric aspirate collected by nasogastric aspiration is recommended only in children and a few adults who do not expectorate sputum [5, 6], its remains one of the clinical specimens routinely collected in our institution from patients with suspected pulmonary tuberculosis. Gastric aspirate has been used for the diagnosis of pulmonary tuberculosis since M. tuberculosis mycobacteria were shown to resist the low gastric pH decades ago [7]. Because nasogastric aspiration is an invasive procedure that is not comfortable for the patient and is potentially harmful, and gastric aspirate is no longer recommended for adults, we aimed to evaluate the contribution of gastric aspirate analysis to the diagnosis of pulmonary tuberculosis in our institution.
Our Mycobacteria Reference Laboratory in Marseilles, France, provides services for the laboratory diagnosis of tuberculosis and non-tuberculous mycobacteria infections for an urban area of one million people. For the diagnosis of pulmonary tuberculosis, our laboratory routinely analyzes sputum specimens collected in sterile containers without preservative and gastric aspirate specimens collected by nasogastric aspiration. Since January 2010, we have also analyzed stool specimens, as previously reported [8]. Laboratory diagnosis is based on the direct examination of clinical specimens after Ziehl–Neelsen staining followed by standard NaOH decontamination (respiratory tract specimens and gastric aspirates) or chlorhexidine decontamination (stool specimens), as previously reported [8], and on the analysis of inoculated BACTEC BD BBL MGIT 7H9 tubes using the non-radiometric BACTEC MGIT 960 apparatus and the accompanying software, version V5.01A (Becton Dickinson, Le Pont-de-Claix, France). The identification of mycobacterial isolates is performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF) as previously described [9], and genotyping is performed by multispacer sequence typing, as previously described [3].
A case of pulmonary tuberculosis was defined as a patient presenting with: (1) clinically compatible systemic and pulmonary signs and symptoms; (2) radiologically compatible signs; and (3) from whom M. tuberculosis was isolated from at least one sputum, gastric aspirate, or stool specimen. The patients were informed that the results of the laboratory investigation could be used in an anonymous publication, and this study was approved by the IFR48 local ethics committee. A Fisher exact test was used to analyze the percentage of detection of M. tuberculosis in the different clinical specimens, with a p-value of <0.05 for statistical significance.
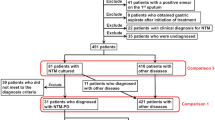
Here, we reviewed the laboratory data for patients diagnosed with pulmonary tuberculosis from January 2010 to April 2012. During this 28-month period, 82 patients met the case definition for pulmonary tuberculosis. The proportion of male patients was 57.3 % (47 males, 35 females), and the patients had a mean age of 46.7 years (standard deviation, 19.45 years; range, 2 months to 91 years). Among these 82 patients, 79 patients yielded at least one M. tuberculosis culture-positive sputum sample. Sputum samples were not available for the other three patients. Nine of 15 (60 %) patients for whom gastric aspirate specimens were available had at least one positive gastric aspirate culture that was positive for M. tuberculosis (Table 1). Twenty-five of 39 (64.1 %) patients for whom stool specimens were available had at least one M. tuberculosis culture-positive stool specimen. The median delay for positive culture did not significantly differ between sputum (14 ± 7 days), stool (15 ± 4.5 days), and gastric aspirate (17 ± 5 days). Among four patients with culture-negative gastric aspirate and stool analysis, one had a culture-positive stool specimen. The difference for culture positivity between gastric aspirate and stools was not significant. The three patients for whom sputum specimens were not available included the 2-month-old patient and two adult patients (Table 1); all three of these patients had at least one M. tuberculosis culture-positive gastric aspirate and one or more M. tuberculosis culture-positive stool specimens (Table 1).
The data presented herein indicate that, in our laboratory, gastric aspirate analysis alone did not contribute to the laboratory diagnosis of pulmonary tuberculosis, as no patient had a culture-positive gastric aspirate and culture-negative sputum and stool specimens. The data presented herein confirm that analyzing gastric aspirate specimens is not useful when a respiratory tract specimen is available for a patient [6]. For the few patients for whom sputum samples were not available, gastric aspirate samples yielded M. tuberculosis in culture. In these patients, however, parallel stool analysis allowed culture-based diagnoses. Altogether, the diagnostic yield of gastric aspirate analysis was lower than that of the stool specimen analysis for the laboratory diagnosis of pulmonary tuberculosis.
The results reported herein differ from those previously reported. Several past studies indicated that gastric aspirate culture was significantly superior to the culturing of stool specimens for the diagnosis of pulmonary tuberculosis [10–12]. However, these studies targeted a pediatric population, whereas we enrolled primarily adult patients. Moreover, these three studies used sodium hydroxide to decontaminate stool specimens; in our laboratory, stool decontamination is performed with chlorhexidine. Chlorhexidine is not active against mycobacteria, including M. tuberculosis [13, 14], and chlorhexidine-based decontamination has been demonstrated to increase the yield of M. tuberculosis [7, 15] and nontuberculous mycobacteria isolated from sputum relative to sodium hydroxide decontamination [16]. A prospective study in our laboratory performed in 2009 confirmed this result for stool samples and enabled us to assert that analyzing stool specimens has good sensitivity in the diagnosis of pulmonary tuberculosis [7].
In conclusion, gastric aspirate is not necessary for the culture-based diagnosis of pulmonary tuberculosis when respiratory tract specimens are available. When no respiratory tract specimens are available, stool specimens are an alternative to gastric aspirate if the laboratory protocol for stool specimens includes chlorhexidine decontamination.
References
World Health Organization (WHO) (2012) Global tuberculosis control: WHO report 2011. WHO, Geneva
McNerney R, Maeurer M, Abubakar I, Marais B, McHugh TD, Ford N, Weyer K, Lawn S, Grobusch MP, Memish Z, Squire SB, Pantaleo G, Chakaya J, Casenghi M, Migliori GB, Mwaba P, Zijenah L, Hoelscher M, Cox H, Swaminathan S, Kim PS, Schito M, Harari A, Bates M, Schwank S, O’Grady J, Pletschette M, Ditui L, Atun R, Zumla A (2012) Tuberculosis diagnostics and biomarkers: needs, challenges, recent advances, and opportunities. J Infect Dis 205(Suppl 2):S147–S158
Djelouadji Z, Arnold C, Gharbia S, Raoult D, Drancourt M (2008) Multispacer sequence typing for Mycobacterium tuberculosis genotyping. PLoS One 3:e2433
World Health Organization (WHO) (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO, Geneva
Pomputius WF 3rd, Rost J, Dennehy PH, Carter EJ (1997) Standardization of gastric aspirate technique improves yield in the diagnosis of tuberculosis in children. Pediatr Infect Dis J 16:222–226
Procop GW, Roberts GD (2011) Laboratory diagnosis and susceptibility testing. In: Schlossberg D (ed) Tuberculosis and nontuberculous mycobacterial infections, 6th edn. ASM Press, Washington DC, pp 66–74
Chierakul N, Anantasetagoon T, Chaiprasert A, Tingtoy N (2003) Diagnostic value of gastric aspirate smear and polymerase chain reaction in smear-negative pulmonary tuberculosis. Respirology 8:492–496
El Khéchine A, Henry M, Raoult D, Drancourt M (2009) Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology 155:2384–2389
El Khéchine A, Couderc C, Flaudrops C, Raoult D, Drancourt M (2011) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS One 6:e24720
Donald PR, Schaaf HS, Gie RP, Beyers N, Sirgel FA, Venter A (1996) Stool microscopy and culture to assist the diagnosis of pulmonary tuberculosis in childhood. J Trop Pediatr 42:311–312
Oberhelman RA, Soto-Castellares G, Gilman RH, Caviedes L, Castillo ME, Kolevic L, Del Pino T, Saito M, Salazar-Lindo E, Negron E, Montenegro S, Laguna-Torres VA, Moore DA, Evans CA (2010) Diagnostic approaches for paediatric tuberculosis by use of different specimen types, culture methods, and PCR: a prospective case–control study. Lancet Infect Dis 10:612–620
Oberhelman RA, Soto-Castellares G, Caviedes L, Castillo ME, Kissinger P, Moore DA, Evans C, Gilman RH (2006) Improved recovery of Mycobacterium tuberculosis from children using the microscopic observation drug susceptibility method. Pediatrics 118:e100–e106
Best M, Sattar SA, Springthorpe VS, Kennedy ME (1990) Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol 28:2234–2239
Rikimaru T, Kondo M, Kondo S, Oizumi K (2000) Efficacy of common antiseptics against mycobacteria. Int J Tuberc Lung Dis 4:570–576
Peres PJ, Gevaudan MJ, Gulian C, de Micco P (1988) Une méthode de traitement des produits pathologiques en vue de l’isolement des mycobactéries. Revue Fr Lab 173:67–74
Ferroni A, Vu-Thien H, Lanotte P, Le Bourgeois M, Sermet-Gaudelus I, Fauroux B, Marchand S, Varaigne F, Berche P, Gaillard JL, Offredo C (2006) Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J Clin Microbiol 44:2237–2239
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonnave, PE., Raoult, D. & Drancourt, M. Gastric aspiration is not necessary for the diagnosis of pulmonary tuberculosis. Eur J Clin Microbiol Infect Dis 32, 569–571 (2013). https://doi.org/10.1007/s10096-012-1776-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1776-6