Abstract
The purpose of this investigation was to enhance the detection of pneumococcal bacteremia cases using the Binax NOW® immunochromatographic test (ICT) on blood culture broth as part of surveillance in two rural Thailand provinces. Blood cultures were collected as clinically indicated from hospitalized patients. ICT was performed on broth from culture bottles flagged as positive by BactT/ALERT® (alarm-positive) but which failed to grow organisms on subculture. During the period May 2005–June 2007, ICT was positive on 43 (24%) of 182 alarm-positive blood cultures with no growth on subculture. Compared to pneumococcal bacteremia cases confirmed by culture, cases detected only by ICT had a longer median time from culture collection to incubation and a longer median time from alarm positivity to subculture, and were more likely to be from patients pretreated with antibiotics. In a subsequent surveillance period (July 2007–December 2009), ICT continued to detect additional pneumococcal cases, but in a lower proportion of samples (7 of 221, 3.2%). Recently, as part of a separate study, ICT applied to uninoculated blood culture broth produced weak-positive results, mandating caution if testing broth from patient blood cultures. The antigen testing of blood culture broth appears to enhance the detection of pneumococcal bacteremia, but a controlled evaluation is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Culture-based detection methods for pneumococcus lack sensitivity and are often not available in resource-limited settings. Binax NOW® is a rapid immunochromatographic test (ICT) to detect pneumococcal antigen in urine (adults) and cerebrospinal fluid [1]. Binax ICT effectively detected pneumococcal antigen from blood culture broth in a laboratory-based study [2], but clinical evaluations are lacking. As part of pneumonia and bloodstream infection surveillance, we sought to enhance pneumococcus detection using the Binax NOW® ICT on blood culture broth from selected patients hospitalized in Thailand.
Materials and methods
ICT use as part of bloodstream infection surveillance
As part of pneumonia and bloodstream infection surveillance in 20 hospitals in two rural Thailand provinces (Sa Kaeo and Nakhon Phanom), blood cultures were collected as clinically indicated [3]. All cultures were processed in the single provincial (i.e., referral) hospital in each province. Cultures collected at community hospitals were transported at 15–30°C within 24 h and processed at provincial hospital laboratories using the BactT/ALERT® 3D system (bioMérieux); the results of single patient blood cultures in bottles optimized for aerobic growth were used for this analysis. Technicians were available daily from 8 am to midnight. Bottles that signaled positive growth (alarm-positive) were subcultured using standard methods [4]. When cultures that alarmed positive yielded no organism on subculture, broth from the culture bottle was tested for the presence Streptococcus pneumoniae antigen by the Binax NOW® ICT following the manufacturer’s instructions for testing urine [2]. Antibiotic use before blood culture was determined by measuring the serum antimicrobial activity using a serum disc assay as previously described [3, 5]. Briefly, a filter paper disc coated with 20 μl of patient serum was placed onto a Mueller–Hinton agar plate inoculated with antibiotic-sensitive S. aureus (ATCC 9144). After 24 h of incubation at 35–37°C, growth inhibition around the disc was considered to be evidence of serum antimicrobial activity.
Statistical analysis
Surveillance data were analyzed for two separate time periods. The primary data analysis was performed in January 2008 in preparation for a meeting presentation, and included blood cultures collected from May 2005 through June 2007. A second analysis was performed for cultures collected from July 2007 through December 2009. Because the frequency of ICT-positive cases differed significantly by time period, the results are presented separately. All analyses were conducted using SPSS version 17.0.
Testing of uninoculated blood culture broth by ICT
As part of a controlled evaluation of ICT testing of blood culture broth, we discovered that broth from some of the uninoculated blood culture bottles gave a faint positive result by ICT. Because this finding has implications for the interpretation of our findings, we present the preliminary data of ICT testing of broth from uninoculated BactT/ALERT® 3D culture bottles (bioMérieux), as well as broth from Bactec bottles (Becton Dickinson BD®, Franklin Lakes, NJ, USA). Before testing, we developed a quantitative scoring system to grade the strength of positive results as follows: 1+ = test line positive but fainter than the positive control line; 2+ = test line positive with similar intensity to the control line; 3+: test line positive with greater intensity than the control line. All ICT results were interpreted by a single technician (P.S.).
Results
Surveillance results
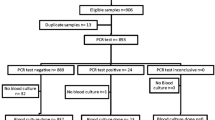
Among 24,173 blood cultures collected from May 2005 through June 2007, 2,915 (12%) were alarm-positive and 66 yielded S. pneumoniae; broth from these culture bottles were all ICT-positive. Of 182 (6%) alarm-positive cultures that yielded no growth on subculture, 43 (24%) were ICT-positive for pneumococcus, 39 of which occurred in Nakhon Phanom province. There was no evidence of clustering by hospital. A non-systematic sample of broth specimens from cultures yielding non-pneumococcal Streptococcus species were tested by ICT, and some false-positives occurred: viridans (5 of 13 tested), mitis (7 of 34), and salivarius (1 of 2).
Compared to cases confirmed by pneumococcal isolation, cases detected only by ICT had a longer median time from culture collection to incubation and a longer median time from alarm positivity to subculture (Table 1). Preculture antibiotic use was more common among pneumococcal cases detected by ICT alone than those with pneumococcal isolation. Cases detected by ICT alone were more likely than culture-confirmed cases to result from cultures collected at community hospitals (vs. provincial hospitals), but there was otherwise no apparent clustering by hospital. No difference was found in the blood volumes cultured. Among 70 cases with clinical information available, cases detected only by ICT were less likely than those with pneumococcal isolation to require mechanical ventilation and have a fatal outcome.
From July 2007 through December 2009, 39,239 blood cultures were performed and 111 culture-confirmed pneumococcal bacteremia cases occurred. Of 5,311 alarm-positive cultures, 365 (6.9%) yielded no growth on subculture, but only 7 (3.2%) of 221 tested were positive by ICT, and these cases clustered in time from April through June 2009 in Nakhon Phanom province only. We saw no evidence of improved pneumococcal isolation rates or decreases in the culture processing times during this time period.
ICT on broth from uninoculated blood culture bottles
We tested broth from 15 uninoculated BacT/ALERT® aerobic culture bottles from five different lots, including 12 pediatric bottles (PF™) optimized for smaller blood volumes. ICT was positive at 1+ for 11 bottles and 2+ for one PF bottle. We also tested four BacT/ALERT® MB™ bottles (two lots) which have broth optimized for mycobacterial growth, and all were positive (1+). Four BD aerobic bottles from two different lots, including two pediatric bottles, were ICT-negative. Two BD bottles optimized for mycobacterial growth were ICT-positive at 1+.
Discussion
Pneumococcal antigen testing of broth from alarm-positive/subculture-negative blood cultures identified potential pneumococcal bacteremia cases that were missed by standard culture methods. Cases identified only by ICT differed from culture-confirmed cases in important ways: longer culture transport times resulting in delayed incubation, delayed subculture after alarming positive, and higher rates of preculture antibiotics. ICT cases clustered in Nakhon Phanom province where distances from community to provincial hospitals are greater, resulting in longer delays between culture collection and incubation. These differences are consistent with the fastidious nature of pneumococcus and support the observation that cases detected only by ICT were real. Cases detected by ICT alone were associated with less severe illness, possibly suggesting a lower bacterial burden or earlier antimicrobial therapy. However, ICT-positivity in uninoculated bottles leaves us unsure whether each case was a true bacteremia case. These findings underscore the challenges of using blood culture alone to diagnose pneumococcus, especially in resource-limited settings.
Alarm-positive/subculture-negative blood cultures have been reported at rates of 1–10%, and previous studies have identified pneumococcus in these specimens by polymerase chain reaction and latex agglutination [6–8]. Limitations to the current study include the lack of confirmatory testing, such as polymerase chain reaction, to rule out false-positives by ICT and determine specificity. Without the ability to determine serotypes of cases detected only by ICT, we could not determine whether the clustering by time and province was related to a particular serotype(s) that may have been difficult to culture. Reasons for the decline in cases confirmed only by ICT in more recent years are not clear; improvements in pneumococcal isolation or culture processing do not easily explain these results. There has been no change in the culture system (BacT/ALERT®) used for surveillance over time.
The ICT testing of broth from patient blood cultures is not a validated approach to confirm pneumococcal disease. ICT-positivity of uninoculated culture bottles confirms that false-positive tests can occur and may be common, depending on the blood culture system. False-positive ICT results occurred consistently on BactT/ALERT bottles from multiple lots, arguing against a spurious finding. False-positives did not occur on broth from aerobic BD® culture bottles, suggesting that a unique component of the BactT/ALERT broth may be responsible. Culture broth formulations are proprietary, so we were unable to explore potential causative agents. While almost all false-positive results were weakly positive, the manufacturer’s instructions state that any visible test line should be considered as positive. Although we did not record quantitative ICT results (i.e., negative, 1+, 2+) in our pneumococcal disease surveillance, 65% (28/43) of pneumococcus cases detected only by ICT during the period May 2005—June 2007 were recorded as “strong positive” by non-standardized qualitative assessment at the time of testing. The Binax® ICT has been validated to confirm pneumococcus in urine from adults and cerebrospinal fluid from all ages of patients, but not on blood or blood culture broth. A full evaluation of rapid antigen testing for this clinical application is underway.
References
Boulware DR, Daley CL, Merrifield C, Hopewell PC, Janoff EN (2007) Rapid diagnosis of pneumococcal pneumonia among HIV-infected adults with urine antigen detection. J Infect 55:300–309
Petti CA, Woods CW, Reller LB (2005) Streptococcus pneumoniae antigen test using positive blood culture bottles as an alternative method to diagnose pneumococcal bacteremia. J Clin Microbiol 43:2510–2512
Baggett HC, Peruski LF, Olsen SJ, Thamthitiwat S, Rhodes J, Dejsirilert S, Wongjindanon W, Dowell SF, Fischer JE, Areerat P, Sornkij D, Jorakate P, Kaewpan A, Prapasiri P, Naorat S, Sangsuk L, Eampokalap B, Moore MR, Carvalho G, Beall B, Ungchusak K, Maloney SA (2009) Incidence of pneumococcal bacteremia requiring hospitalization in rural Thailand. Clin Infect Dis 48(Suppl 2):S65–S74
Perilla MJ, Ajello G, Bopp C, Elliott J, Facklam R (2003) Manual for the laboratory identification and antimicrobial susceptibility testing of bacterial pathogens of public health importance in the developing world. Centers for Disease Control and Prevention (CDC), Atlanta, GA
Rhodes J, Hyder JA, Peruski LF, Fisher C, Jorakate P, Kaewpan A, Dejsirilert S, Thamthitiwat S, Olsen SJ, Dowell SF, Chantra S, Tanwisaid K, Maloney SA, Baggett HC (2010) Antibiotic use in Thailand: quantifying impact on blood culture yield and estimates of pneumococcal bacteremia incidence. Am J Trop Med Hyg 83:301–306
Khoosal MWJ, de Gouveia L, du Plessis M, Manqalaza F, Karstaedt AS, Madhi SA (2005) Usefulness of latex antigen test in improving the sensitivity of detection of pneumococcus from blood culture. In: Program and abstracts of the 1st Joint Congress of the Federation of Infectious Diseases Societies of Southern Africa, Sun City, South Africa, July 2005
Karahan ZC, Mumcuoglu I, Guriz H, Tamer D, Balaban N, Aysev D, Akar N (2006) PCR evaluation of false-positive signals from two automated blood-culture systems. J Med Microbiol 55:53–57
Saha S, Darmstadt G, Naheed A, Arifeen S, Islam M, Fatima K, Breiman R, Sack D, Hamer D (2011) Improving the sensitivity of blood culture for Streptococcus pneumoniae. J Trop Pediatr 57:192–196
Acknowledgments
For their contributions to this work, we acknowledge Sathapana Naorat, Prabda Prapasiri, Sununta Henchaichon, Prasong Srisaengchai, Thantapat Akarachotpong, Puangtong Tungpruchayakul, Pornpak Khunatorn, Pattraporn Klanjatturat, and Wanna Wongjindanon from the International Emerging Infections Program; Denchai Sornkij, Nakhon Phanom Provincial Health Office; Peera Areerat, Sa Kaeo Provincial Health Office; and Pokasem Sirinarm and Pichai Thongtaradol from the Thailand Ministry of Public Health. Thanks go to Scott Dowell, Karen Miernyk, and Matt Moore from the U.S. Centers for Disease Control and Prevention (CDC) for their technical input. Support for this project was provided by the CDC Foundation and the Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP), which was funded by the GAVI Alliance and is based at the Johns Hopkins Bloomberg School of Public Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baggett, H.C., Rhodes, J., Dejsirilert, S. et al. Pneumococcal antigen testing of blood culture broth to enhance the detection of Streptococcus pneumoniae bacteremia. Eur J Clin Microbiol Infect Dis 31, 753–756 (2012). https://doi.org/10.1007/s10096-011-1370-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1370-3