Abstract
The objective of this investigation was to verify the hypothesis that the presence of lower airway bacterial colonization (LABC) can be a stimulating factor of airway inflammation, more frequent exacerbation, and impact on pulmonary function, independent of current tobacco smoking in the stable phase of chronic obstructive pulmonary disease (COPD). A total of 46 ex-smokers with moderate to severe COPD, 19 healthy non-smokers, and 17 ex-smokers without COPD were included in this study. Their sputum specimens were collected at the first baseline visit and at the second visit after a follow-up of one year. The samples were analyzed for bacterial growth by culture, and the levels of interleukin (IL)-6, IL-8, and tumor necrosis factor alpha (TNF-α) were measured by enzyme-linked immunosorbent assay (ELISA). The frequencies of exacerbations and pulmonary function were compared at visit 2. At visit 1, 37.0% (17/46) were found to have LABC with bacterial loads ≥106 CFU/ml in their sputum specimens. Haemophilus influenzae was the predominant pathogenic organism isolated. IL-8, IL-6, and TNF-α in these patients’ sputum were significantly higher than those without LABC (p < 0.05). It was the presence of LABC that contributed to the significantly elevated IL-8 and IL-6 at the 1-year period (p < 0.05). LABC was also associated with significantly increased frequencies of exacerbations and declined forced expiratory volume in 1 s (FEV1) (p < 0.05). LABC was documented in a subpopulation of stable COPD patients; it may be responsible for the deterioration of pulmonary function of COPD patients by promoting airway inflammation and/or increased frequency of exacerbations independently of tobacco smoking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually associated with an abnormal inflammatory response of the lungs to noxious particles or gases and it is usually progressive [1]. There are two phases in COPD patients: the stable status and the acute exacerbation, the latter emerging from the former. Acute exacerbation COPD (AECOPD) is defined as the worsening of at least two respiratory symptoms, i.e., cough, expectoration, wheezing, dyspnea on exertion, and chest oppression, which are beyond normal day-to-day variations. This is usually acute in onset and may warrant additional treatment [2]. Airway inflammation and AECOPD are now being recognized as important features of the natural history of COPD, and have important implications for health-related quality of life [3]. Also, they are important causes of morbidity and mortality [4]. The pathogenetic mechanism of the airway inflammatory process is complicated, although cigarette smoking is the main pathological driver of COPD. Besides, other factors may be involved which can explain why only a proportion of cigarette smokers develop COPD [5]. Despite the rate of decline of pulmonary function in early COPD, it is often normalized after abstinence of smoking. Still, many ex-smokers with moderate to severe COPD show persistent symptoms and recurrent exacerbations, suggesting that there must be an additional inflammatory stimulus involved in the persistent inflammation [6].
Lower airway bacterial colonization (LABC) was often present in COPD patients in their stable status [7, 8], and was hypothesized to be a potential stimulus for the airway inflammation [8, 9]. Some evidence showed that LABC was associated with higher levels of airway inflammatory cytokines in the sputum, increased frequency of exacerbations, and accelerated decline in pulmonary function [10–12]. However, all of those studies included smoking subjects only, resulting in the interference of proinflammatory effects of smoking with LABC. Furthermore, only a single feature of LABC, AECOPD, airway inflammation, and pulmonary function had been investigated in most of them. In this study, we aimed to evaluate the effects that are limited to ex-smokers only, and attempt to give a better assessment of these factors.
We hypothesize that LABC is a potential stimulus to airway inflammation or frequency of exacerbations in COPD independently of current tobacco smoking. Hence, we defined the quantitative bacterial load and compared the levels of interleukin (IL)-6, IL-8, and tumor necrosis factor alpha (TNF-α) in the sputum, the frequency of AECOPD, and pulmonary function at the stable status, one year before and after the abstinence of smoking in patients with COPD and healthy subjects who did or did not smoke.
Patients and methods
Study subjects
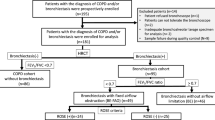
The study protocol was approved by the ethics committee of The First People’s Hospital Affiliated to Shanghai Jiaotong University. Three groups of volunteers were included. Group 1 consisted of ex-smokers with COPD. In addition, 46 patients with moderate to severe COPD in their stable status were recruited. The inclusion criteria were: (1) forced expiratory volume in 1 s (FEV1) <80% predicted value according to age and height; (2) β2 agonist reversibility on predicted FEV1 <15% and/or <200 ml; (3) FEV1/forced vital capacity (FVC) <70%; (4) subjects in stable status without evidence of exacerbation for at least 6 weeks; (5) at least one episodic attack of exacerbation in the previous year; (6) abstinence of smoking for at least 2 years before enrollment; (7) signed informed consent. The exclusion criteria were: (1) coexistent asthma, bronchiectasis, carcinoma of bronchus, or other serious respiratory disease; (2) use of antibiotic or systemic steroid therapy in the four weeks preceding the enrollment; (3) having a history of immune diseases. Exacerbations were diagnosed according to the previous consensus definition of AECOPD. Group 2 and group 3 enrolled ex-smokers without COPD and healthy non-smokers, respectively, which were confirmed by spirometry and high-resolution computed tomography (HRCT).
Study procedures
Visit 1 and visit 2 were at baseline and 1 year after enrollment, respectively. At visit 1, measurements were taken for height, weight, and HRCT in the three groups; arterial blood gas analysis for partial pressure of oxygen and carbon dioxide was only measured in group 1. Sputum collection for quantitative bacteriology analysis, bacterial culture, cytokines level measurement, and pulmonary function test were performed at visit 1 among the patients in the three groups and at visit 2 follow-up in group 1. At visit 2, the frequency of episodic attacks of exacerbation was recorded in group 1.
Sputum collection and quantitative analysis of bacteriology
Subjects were instructed to rinse their mouths with water before expectorating the sputum into a sterile bottle. If volunteers were unable to produce sputum, then they used 3% sterile saline for 10 min for sputum induction. All sputum samples were sent to the laboratory for analysis as soon as possible. After being washed with saline solution, the sputum samples containing less than ten squamous epithelial cells and more than 25 leukocytes per low-power field were accepted for processing. Otherwise, sputum should be recollected from the patient immediately. Sputum was separated from contaminating saliva by mixing with 0.9% saline five times. Sputolysin (Oxoid Ltd., Poole, England) with a weight being equivalent to that of the sputum sample was then added and each sample was diluted (1:106). These dilutions of the homogenized sample were tested for microorganisms according to standard methods. After incubation, bacterial colonies were counted and subcultured for identification by standard methods. Potential pathogenic bacteria (PPB) included Haemophilus spp., Streptococcus pneumoniae, Staphylococcus aureus, Moraxella catarrhalis, Pseudomonas aeruginosa, and gram-negative enteric bacteria [13]. A threshold concentration of 106 CFU/ml was used to define significant growth of PPB [14].
Sputum samples for measurements of TNF-α, IL-6, and IL-8
After mixing sputolysin with the sputum sample, the whole mixture was centrifuged at 2,000 rpm at 4°C for 10 min, which resulted in a cell pellet and supernatant fraction. The supernatants were decanted and stored at –80°C. All cytokines were detected by commercial enzyme-linked immunosorbent assays (ELISAs) (R&D Company, USA). The details are provided in the online supplement and all values are expressed in pg/mL.
Frequency of exacerbations
Progressive worsening of the condition with acute-onset or day-to-day variations beyond normal might warrant additional treatment and were required to report to us immediately. We also followed up the occurrence of the exacerbation once every two months.
Pulmonary function
Pulmonary function examination was carried out with a rolling sealed spirometer (MS-105, Jaeger Company, GM), between 9 am and 11 am, 30 min after 200 μg of the bronchodilator, salbutamol, was administered via a metered dose inhaler. At least three spirometry readings were taken.
Statistical analysis
Data analyses were performed using SPSS for Windows™ version 11.5. Normally distributed data are presented as mean ± standard deviation (SD) and skewed data as median values. Continuous variables with normal distributions were compared by a two-tailed unpaired t-test, while those with abnormal distributions were compared by the Wilcoxon signed-rank test. Data comparison of more than two groups was done by one-way analysis of variance (ANOVA). Correlations were assessed using the Spearman correlation coefficient. p < 0.05 was taken to be statistically significant.
Results
Subject characteristics
A total of 90 patients were enrolled. Eight did not complete the study: three moved to another city, two withdrew from the study, two re-smoked during the study, and one died of a comorbid illness.
Among the 82 subjects enrolled in the subsequent analyses, 46 were recruited into group 1, including 38 males and eight females. All patients were categorized according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD); 24 patients in stage 2 (52.1%) and 22 patients in stage 3 (47.9%) were recruited. Seventeen and 19 subjects were enrolled into groups 2 and 3, respectively. The baseline physiologic characteristics of the patients are summarized in Table 1. There were no statistically significant differences between the patients among these three groups regarding age and body mass index (BMI).
Sputum bacterial loads and isolates
The microbiological results of group 1 at visit 1 indicated that at the stable stage, 17/46 sputum samples (37.0%) yielded a positive culture of PPB based on bacterial colonies (>106 CFU/ml), compared with 0/17 (0%) subjects of group 2 and 1/19 (2.2%) subjects of group 3 (p < 0.001). The dominant pathogens recovered at visit 1 are shown in Table 2.
Cytokine levels in sputum samples
The cytokine levels of patients in the three groups are presented in Table 3. The levels in the sputum of subjects in group 1 were significantly higher than those in the other two groups (p < 0.001).
Relevance of LABC and airway inflammation in COPD
Based on the PPB findings in sputum samples, we categorized the subjects in group 1 into subgroups with (n = 17) or without LABC (n = 29). There were no statistical differences between the two groups regarding the age, BMI, pulmonary obstruction, and arterial blood gas analysis (Table 4). Sputum IL-8, IL-6, and TNF-α were found to be higher in the COPD patients with LABC compared with those patients without LABC (p < 0.05) at visit 1 (Fig. 1). One year afterwards, the level of sputum IL-8 and IL-6 increased significantly in the subgroup with LABC (Table 5).
Comparison of sputum cytokine level among patients with chronic obstructive pulmonary disease (COPD) colonized (COPD, with LABC) and not colonized with potential pathogenic bacteria (COPD, without LABC), smokers, and non-smokers. The horizontal bars represent the mean ± 95% confidence interval values. a Interleukin-8 (IL-8) level (pg/mL). b Interleukin-6 (IL-6) level (pg/mL). c Tumor necrosis factor alpha (TNF-α) level (pg/mL)
Effect of LABC on the frequency of AECOPD and pulmonary function
There was no difference in the change of FEV1 between patients with and without LABC in both visit 1 and visit 2 (Table 6). However, patients with LABC were associated with a significantly increased frequency of exacerbations (p = 0.04) and decline of FEV1 (p = 0.035) (Fig. 2). The deterioration of pulmonary function correlated positively with the frequency of exacerbations (p < 0.05) (Fig. 3).
Discussion
Do COPD patients have LABC during their stable status? Does LABC contribute to airway inflammation and the pathogenesis of AECOPD? In contrast to the sterile airways in normal human beings, bacteria had been isolated in a relatively high proportion of patients with clinically stable COPD. COPD patients with active smoking and progressive airway obstruction had bacteria identified in the lower airways, owing to impairment of the pulmonary defense system (including disruption of ciliary function, hypersecretion of mucus, impaired mucociliary clearance, and decreased phagocytic function of macrophages and neutrophils). As a consequence, microbial pathogens could persist in the lower airway in COPD patients [15], indicating the presence of LABC. LABC has been shown to increase when smoking more cigarettes and airway obstruction worsens [16]. In our study, the subjects enrolled were restricted to ex-smokers, to demonstrate whether LABC still persisted and had potential relevance to airway inflammation, frequency of exacerbation, and pulmonary function changes. Meanwhile, in order to minimize the risk of contamination, we used quantitative cultures of sputum as the gold standard. During the study, we strictly abided by the regulation of sputum sample collection and transport. Only microorganisms forming >106 CFU/ml were regarded as positive. For the measurement of the levels of related cytokines, it was demonstrated that threshold colonies >105–106 CFU/ml can be defined as LABC [17]. The potential pathogens in the sputum samples of our study were predominantly H. influenzae, which was similar to those found in many prior studies of sputum and lower airway bacteriology among active smokers [7]. This indicated that a subgroup of patients with COPD was prone to colonization of PPB without persistent stimulation of tobacco smoking.
Furthermore, on defining the levels of IL-8, IL-6, and TNF-α, an increase of IL-8 was shown in several studies in the sputum and bronchoalveolar lavage (BAL) of COPD patients compared with smokers without COPD [3, 18]; it appeared to be the key chemokine mediator of neutrophilic airway inflammation in COPD [19]. IL-6 and TNF-α, as the important proinflammatory cytokines, were increased in COPD and appeared to promote and amplify the inflammation, leading to the increased expression of multiple inflammation-related genes [20].
Though the significance of LABC in terms of the development and progression of airway inflammation in COPD has not been precisely defined, recent data demonstrated that the presence of LABC can result in a series of important pulmonary pathologic changes, including activation of host defense with the release of inflammatory cytokines such as IL-8, IL-6, and TNF-α, and subsequent neutrophil recruitment [17]. Chung [3] showed that bacterial colonization in patients with stable COPD was associated with increased levels of IL-8, IL-6, and TNF-α in the sputum. Although previous studies had shown the association between LABC and airway inflammation in COPD, they were limited by the confounding effect of the smoking factor [13, 21–23].
In the present study, sputum levels of IL-8, IL-6, and TNF-α were observed to be higher in the abstinent patients with LABC at the stable stage than those in patients without LABC. The differences were significant and no significant differences were noted among age, pulmonary function, arterial blood gas analysis, and ex-smokers between the two groups, indicating that bacterial products might affect the neutrophils migration and modulation of airway inflammation independently. At the same time, we used ex-smokers and non-smokers without COPD as controls to confirm that there had been no difference in the level of inflammation factors between them. Meanwhile, we followed up the patients to see if there were any alterations in chronic inflammation during the one-year period. We found that the levels of IL-8 and IL-6 in the LABC subgroup increased significantly, but no differences in the subgroup without LABC were documented. LABC generated a continuous and potent inflammatory effect independent of smoking.
Bhowmik et al. [24] showed that patients with frequent exacerbations had increased levels of IL-6 and IL-8. However, some investigators did not find such differences [25]. Nevertheless, some recent studies also showed that there seems to be a link between the frequency of exacerbations and IL-8 levels in BAL samples [26] and LABC in sputum samples [11]. In the present study, we found that the presence of bacterial colonization in the stable status was associated with increased frequency of exacerbations, indicating that LABC was an important contributor to frequent exacerbations, even with the coexistence of absence of smoking.
As a prospective study, unlike a cross-sectional and retrospective study, we followed up patients’ pulmonary function, though no differences in the absolute value of FEV1 between the two subgroups at both visit 1 and visit 2 was found. However, when we focused on the decrement of FEV1, significant differences were found which indicated that LABC contributed to the decline of pulmonary function. Additionally, increased frequency of exacerbation was associated with worsened pulmonary function, which was shown by logistic regression analysis. This means that recurrent exacerbations might be an important factor for the deterioration of pulmonary function.
In conclusion, patients with moderate to severe COPD have LABC even at the stable status. LABC is recognized as an important factor in the pathogenesis of airway inflammation and AECOPD. Among the bacterial pathogens colonizing at the stable status, H. influenzae was the most frequently isolated pathogen. Both sputum culture and bacterial colony counts could be associated with the severity of LABC. The bacterial colonization of the tracheobronchial tree, the increase in the levels of airway IL-8, IL-6, and TNF-α at the stable status, as well as the increase of frequency of acute exacerbation and decline in FEV1 indicate that LABC can be an important stimulus impacting the airway inflammation and frequency of AECOPD. All of these observations are involved in the deterioration of pulmonary function. Methods to reduce or eradicate the LABC should be included in future interventions. Whether airway inflammation could lead to aggravation and frequent acute exacerbations, and whether LABC may induce systemic inflammatory response in COPD patients would need further investigation.
References
The Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of COPD: 2003. NHLBI/WHO (GOLD) Workshop Report, NIH publication (2003). United States Department of Health and Human Services
Adams SG, Melo J, Luther M et al (2000) Antibiotics are associated with lower relapse rates in outpatients with acute exacerbations of COPD. Chest 117:1345–1352
Chung KF (2005) Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4:619–625
Lopez AD, Shibuya K, Rao C et al (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27:397–412
Chung KF, Adcock IM (2008) Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur Respir J 31:1334–1356
Anthonisen NR, Connett JE, Murray RP (2002) Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 166:675–679
Aaron SD, Angel JB, Lunau M et al (2001) Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163:349–355
Sethi S, Murphy TF (2001) Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin Microbiol Rev 14:336–363
Barnes PJ (2004) Small airways in COPD. N Engl J Med 350:2635–2637
Banerjee D, Khair OA, Honeybourne D (2004) Impact of sputum bacteria on airway inflammation and health status in clinical stable COPD. Eur Respir J 23:685–691
Patel IS, Seemungal TAR, Wilks M et al (2002) Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 57:759–764
Wilkinson TM, Patel IS, Wilks M et al (2003) Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167:1090–1095
Soler N, Ewig S, Torres A et al (1999) Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J 14:1015–1022
Monsó E, Rosell A, Bonet G et al (1999) Risk factors for lower airway bacterial colonization in chronic bronchitis. Eur Respir J 13:338–342
Sohy C, Pilette C, Niederman MS et al (2002) Acute exacerbation of chronic obstructive pulmonary disease and antibiotics: what studies are still needed? Eur Respir J 19:966–975
Prieto A, Reyes E, Bernstein ED et al (2001) Defective natural killer and phagocytic activities in chronic obstructive pulmonary disease are restored by glycophosphopeptical (Inmunoferón). Am J Repir Crit Care Med 163:1578–1583
Noguera A, Batle S, Miralles C et al (2001) Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 56:432–437
Yamamoto C, Yoneda T, Yoshikawa M et al (1997) Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest 112:505–510
Sethi S, Maloney J, Grove L et al (2006) Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173:991–998
Barnes PJ (2009) The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 41:631–638
Wilkinson TM, Patel IS, Wilks M et al (2003) Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Repir Crit Care Med 167:1090–1095
Stockley RA, O’Brien C, Pye A et al (2000) Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest 117:1638–1645
Fuke S, Betsuyaku T, Nasuhara Y et al (2004) Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 31:405–412
Bhowmik A, Seemungal TAR, Sapsford RJ et al (2000) Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 55:114–120
Gompertz S, Bayley DL, Hill SL et al (2001) Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax 56:36–41
Tumkaya M, Atis S, Ozge C et al (2007) Relationship between airway colonization, inflammation and exacerbation frequency in COPD. Respir Med 101:729–737
Acknowledgments
We thank the staff of the Clinical Microbiology Department of the Hospital for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, M., Li, Q., Zhang, XY. et al. Relevance of lower airway bacterial colonization, airway inflammation, and pulmonary function in the stable stage of chronic obstructive pulmonary disease. Eur J Clin Microbiol Infect Dis 29, 1487–1493 (2010). https://doi.org/10.1007/s10096-010-1027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-010-1027-7