Abstract
In order to improve invasive pulmonary aspergillosis (IPA) diagnosis, a real-time polymerase chain reaction (PCR) assay detecting Aspergillus spp. was developed. Its detection limit reached 2–20 conidia. The retrospective evaluation on 64 bronchoalveolar lavage (BAL) fluids from 57 patients at risk for IPA, including 20 probable and five proven IPA patients, revealed a 88% or 38% sensitivity in direct examination (DE)/culture-positive or culture-negative BAL, respectively, whereas galactomannan (GM) sensitivity reached 88% or 58%, respectively. Influence on the Aspergillus-PCR yield of BAL fluid volume, cellular count and DNA content (evaluated by human β-globin quantification) was assessed. Significantly higher β-globin levels were detected in Aspergillus PCR-positive (median 5,112 pg/μl) than negative (median 1,332 pg/μl) BAL fluids, suggesting that the β-globin level could reflect BAL yields and DNA extraction. Using β-globin for the interpretation of fungal PCR could improve the negative predictive value of this test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive pulmonary aspergillosis (IPA) is a life-threatening infection, occurring mostly in immunocompromised patients. Aspergillus fumigatus is the most common species to cause aspergillosis, but A. flavus, A. terreus and A. niger are also fairly common forms of Aspergillus infection [1]. Diagnosis is often difficult, based on host factors, clinical and radiological findings, and mycological criteria. The European Organization for Research and Treatment of Cancer (EORTC) and the Mycosis Study Group (MSG) have defined three levels for invasive fungal infections, “possible,” “probable” and “proven,” giving a rational basis for the evaluation of treatments and radiological or biological diagnosis tools [2].
Currently, microbiological tools have difficulty in providing a sensitive and early diagnosis of IPA [3, 4], whereas these two criteria are essential for adapted antifungal therapy and improved prognosis. Only positive direct examination (DE) and culture of pulmonary samples obtained by sterile procedures (lung or transbronchial lung biopsies) can confirm the IPA diagnosis and allow identification at the species level, leading to appropriate antifungal therapy. However, these samples, which require invasive procedures for sampling, lack sensitivity, especially if the patient received empirical antifungal treatment [5]. Galactomannan (GM) detection in serum by enzyme-linked immunosorbent assay (ELISA) has a variable sensitivity (29–100%), depending on the host group (immunocompromised or not, probability of IPA in patients included, antifungal treatment) [3, 6, 7]. The specificity of GM generally reaches over 90%, but false-positives can occur, specifically when patients received antibiotics such as piperacillin/tazobactam [6, 8]. Cross-reactivity with other fungal species have also been reported [6, 9]. In bronchoalveolar lavage (BAL), GM detection could lead to an earlier diagnosis of IPA in patients with haematological malignancy, yielding an increased sensitivity when compared to serum, e.g. 85–100% versus 47% [10].
In order to improve IPA diagnosis, molecular methods have been recently developed [11–16]. They can either be specific to one fungal species (mainly A. fumigatus) or target several species (panfungal polymerase chain reaction [PCR]), detecting fungal DNA in serum/blood, biopsies or BAL samples. In addition to being helpful in IPA diagnosis, DNA amplification from biological samples allow species identification when culture remains negative [11–16]. In BAL, which is commonly used for the diagnosis of opportunistic infections in immunosuppressed patients with pulmonary infiltrates, Aspergillus DNA detection yields high sensitivity (67–100%) and specificity (80–100%) [15–22], and has been shown to be clinically relevant for the diagnosis of IPA [23]. However, the features of the BAL fluid could influence fungal detection, as in the diagnosis of alveolar haemorrhage, or interstitial lung diseases, for which the influence of BAL quality on diagnosis has been reported [24], and, consequently, criteria for the selection of suitable samples representative of the lower respiratory tract were defined for the diagnosis of these diseases [25].
With the aim of exploring how a low-quality BAL could result in a decreased sensitivity/specificity of diagnosis tests, we retrospectively evaluated the detection of Aspergillus spp. by real-time PCR on BAL from patients with haematological malignancy. We compared PCR to other available IPA diagnosis tools (DE, culture and GM detection) and determined how PCR can contribute to improve IPA diagnosis sensitivity when BAL quality using cytological analysis and the evaluation of human DNA content by β-globin gene molecular quantification are assessed.
Materials and methods
Patients
Sixty-four BAL from 57 patients referred between December 2000 and February 2004 in the Hematology Department of Lille University Hospital were retrospectively analysed. All of these 57 patients were at risk for IPA, i.e. neutropaenic, with persistent fever under broad-spectrum antibiotics treatment, graft-versus-host disease (GVHD), immunosuppressive agent or corticosteroid treatment. According to the criteria of the EORTC/MSG [2], 25 of 57 patients (yielding 32 BAL for analysis) were classified by a physician as probable (n = 20) or proven (n = 5) IPA, and the 32 remaining patients (32 BAL) were considered as “no IPA” patients (Table 1).
BAL sampling and conventional analysis
In our institution, BAL is performed using a 150–200-ml volume of sterile saline. The reaspirated volume is noted and the sample divided into two parts. One part is sent to the Pathology Department, where the cytological analysis is performed. Cytological data (cellularity and formula) were collected when available. The second part of the BAL is sent to the Microbiology Department for bacteriological, virological and mycological analysis. For mycological conventional and molecular analysis, BAL samples were then subdivided into two fractions.
The conventional analysis included a centrifugation step. A pellet was used for DE after staining with toluidine blue O and May-Grünwald-Giemsa and fungal cultures on Sabouraud agar medium. The BAL supernatant was used to perform the Aspergillus GM antigen test (Platelia Aspergillus EIA, BioRad®), using index ≥1 as positive [10, 26]. BAL GM was used as an EORTC/MSG criteria to classify IPA patients [2].
BAL molecular analysis
The molecular analysis included a centrifugation step and a DNA extraction from the pellet as previously described [27]. Briefly, after centrifugation, the supernatant was discarded and DNA was extracted from 200 μl of BAL pellet using the QIAamp DNA Mini Kit (Qiagen SA, Courtaboeuf, France), following the manufacturer’s instructions, and eluted in 200 μl of sterile water. DNA extracts were stored at −20°C until analysis.
The detection of Aspergillus spp. by real-time PCR was performed with a 5-μl DNA extract on a LightCycler instrument (Roche Molecular Biochemicals, Meylan, France), as previously described [11, 28]. Fluorescence curves were analysed with LightCycler software version 3.5. A standard curve was generated by running eight positive controls containing A. fumigatus IPP 22–7994 genomic DNA (from 1 fg/μl to 10 ng/μl). Two samples (one sterile water submitted to extraction and one PCR mix control) were included as negative controls. Two DNA amplifications were initially performed on each BAL sample. An amplification was considered to be positive when the Cp value was ≤43 [29]. When both replicates were positive, the sample was considered to be positive. When only one of the replicates was positive, DNA amplification was performed again twice: 2/4 or 3/4 PCR-positive results yielded a PCR-positive sample.
PCR inhibitor control was performed using 50 picograms (pg) of A. fumigatus IPP 22–7994 added to separate PCR mixtures containing 5 μl of each BAL fluid. Such quantities of exogenous Aspergillus DNA was supposed to minimise the interference with endogenous fungal DNA when PCR BAL was positive. The amplification kinetics were compared to the kinetics of the 50-pg standard (Cp values of each BAL sample versus the mean of 50-pg standard replicate Cp values). A difference of over three PCR cycles (1 log) was considered as significant inhibition.
In order to evaluate human DNA content in the BAL samples, a quantitative human β-globin PCR was performed using the Control Kit DNA (Roche Molecular Biochemicals, Meylan, France), according to the manufacturer’s instructions; the results were expressed as pg of human DNA per μl.
Performance of the combined extraction–amplification method
The analytical sensitivity of the combined extraction–amplification method was evaluated using serial dilutions of A. fumigatus conidia (104 to 101 conidia/ml) in pooled serums from patients not at risk for IPA (absence of Aspergillus antigen [GM] or antibodies had been checked). For each dilution, DNA extraction was performed twice in two independent experiments, from 200 μl of serum with conidia (corresponding to 2,000, 200, 20 and 2 conidia), using the QIAamp DNA Mini Kit (Qiagen SA, Courtaboeuf, France), as described above. One extraction control (200 μl of serum without conidia) was included in each series. DNA extracts were then analysed in duplicate using the real-time PCR method described above.
Assessment of BAL quality
As BAL composition (alveolar macrophages, ciliated cells, mucus, total cellularity etc.) reflects BAL quality [25], we analysed BAL using the following indicators: (i) volume recovered for analysis, which could be associated with the sample quality [30]; (ii) total cellularity and detailed cell count, which reveal potential contamination by proximal airways or terminal bronchioles [25]; and (iii) human β-globin gene quantification by real-time PCR, which could reflect high cellularity of the BAL and confirm a good quality of DNA extraction, required to perform an efficient PCR reaction. For cell count analysis, alveolar macrophages under 80%, lymphocytes over 15% and neutrophils or bronchial cells over 10% were considered as abnormalities in the BAL formula according to the published data [25, 30].
Statistical methods
The Wilcoxon test was used to compare the duration of the antifungal treatment, BAL volume recovered, total cellularity and the β-globin level medians between IPA patient subgroups classified according to their positive or negative result for DE/culture, GM and PCR. A P-value equal to or less than 0.05 was considered to be statistically significant.
Results
Analytical sensitivity of the DNA extraction and amplification methods
First, we quantified the threshold for fungal DNA detection by real-time PCR using serial dilutions of A. fumigatus genomic DNA (from 1 fg/μl to 10 ng/μl), which reached 1–10 fg/μl, according to previous data [11]. Secondly, the ability and reproducibility of the QIAamp DNA Mini Kit extraction method to extract fungal DNA was evaluated using serial dilutions of A. fumigatus conidia. The median Cp values of the 104, 103 and 102 conidia/ml dilutions (n = 4) reached 35.6, 38.0 and 41.4, respectively, yielding a linear increase in the median Cp as a function of the number of conidia added for extraction (R 2 = 0.990, Fig. 1). The 101 conidia/ml dilution (corresponding to the DNA extraction of two conidia, Fig. 1) exhibited positive DNA amplification in 2 out of 4 replicates (median Cp value of 41.4), suggesting that our combined DNA extraction–amplification method reached a 101–102 conidia/ml (2–20 conidia, Fig. 1) overall sensitivity, and was similar to the most sensitive methods [31, 32].
Combined extraction–amplification method for the determination of the detection limit. Median Cp values were calculated for each Aspergillus fumigatus conidia dilution (104, 103, 102 and 101/ml) and were related to the corresponding number of conidia extracted in 200 μl of serum (2,000, 200, 20 and 2 conidia, respectively)
Contribution of real-time PCR to IPA diagnosis
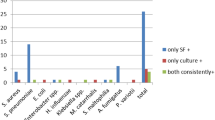
The positive results of each mycological test (DE/culture, GM and PCR) are summarised in Table 2, with the performance of either PCR or GM when they are combined with other tests. The sensitivity of PCR was higher when other tests were also positive: 88% versus 38% in DE/culture-positive BAL, and 57% versus 36% in GM-positive BAL. The sensitivity of GM was similar to that of PCR in DE/culture-positive BAL (88%), but higher in DE/culture-negative BAL (58% versus 38%, Table 2). Both PCR and GM were independently negative in a BAL with positive culture (patients nos. 5 and 6, Table 1, respectively). Within the 26 BAL with negative culture, the detection of Aspergillus spp. DNA by PCR in 11 BAL (patients nos. 7–12, 20–22, Table 1) was the only test supporting the Aspergillus infection (DE and GM are not specific to Aspergillus spp.). Within the ten BAL from seven patients with proven or probable IPA that were negative for DE/culture and GM (patients nos. 3, 20–25, Table 1), three BAL yielded a positive PCR result (patients nos. 20 and 22, Table 1). For these patients, PCR was the only test supporting the diagnosis of IPA in BAL. None out of the 32 BAL from patients without suspected IPA showed a PCR-positive result. A variation under 1 PCR cycle between BAL sample Cp values when 50 pg is added and the mean of 50-pg standard replicate Cp values (25.05 ± 0.22 Cp) confirmed the absence of PCR inhibitors.
Influence of antifungal treatment on the performance of Aspergillus tests
Because of host clinical risks, most patients received antifungals before BAL was performed. The number of molecules used and the total duration of the treatment, which ranged from 0 to 67 days, are indicated in Table 1. The treatment duration medians between negative and positive subgroups for each test (DE/culture, GM detection, PCR) were compared and yielded no significant difference for any of the three tests (Table 3, P > 0.05).
Alveolar micro-environment: evaluation of BAL quality and influence on Aspergillus detection
The volume recovered for analysis, total cellularity, detailed cell count (when available) and human β-globin gene quantification by real-time PCR were used as indicators of BAL quality (Table 1). The range variations and medians were compared when patients are classified according to their IPA status (proven or probable IPA versus no IPA, Table 3). BAL abnormalities of cell analysis were more frequent in patients without IPA, especially due to an increase in lymphoplasmocyte or neutrophil counts (Table 3), and probably related to the underlying haematological disease or to a non-fungal infection (e.g. bacterial). In terms of BAL quality, there was no significant difference between the proven or probable IPA and no IPA patient groups (P > 0.05, Table 3), even if large variations of volume recovered, total cellularity and β-globin individual values were observed.
In patients with proven or probable IPA, the medians of the volume recovered, total cellularity and β-globin levels between negative and positive subgroups for each test (DE/culture, GM detection, PCR) were compared (Table 3). We observed a significantly higher β-globin level in DE/culture- and PCR-positive subgroups (P ≤ 0.05, Table 3), reaching 41,573 pg/μl (1,368 pg/μl in DE/culture-negative samples) and 5,116 pg/μl (1,332 pg/μl in PCR-negative samples), respectively. All BAL with β-globin levels over 15,690 pg/μl were Aspergillus PCR-positive (six BAL, patients nos. 1, 2, 7, 8, 10 and 20, Table 1). Few samples with low β-globin levels and which were fungal PCR-positive have also been observed (e.g. patients nos. 3, 11 or 12, Table 1). The volume recovered and cellularity were not significantly different in the positive and negative subgroups for any of the three tests (P > 0.05, Table 3).
Discussion
Contribution of real-time PCR to IPA diagnosis
The retrospective evaluation of a real-time PCR assay on BAL in patients with haematological malignancy at risk for IPA (n = 57; 64 BAL) confirmed previous analytical sensitivity [11], which reached 1–10 fg/μl, similarly to the most accurate real-time PCR or nested PCR assays targetting the classical rRNA genes [13–15, 33, 34] or other mitochondrial genes [16]. Our combined DNA extraction–amplification method was able to detect 2–20 conidia. Our detection limit was similar to the detection limit of 1–10 conidia reported when A. fumigatus DNA extraction methods were used [31, 32] and higher than a recently reported 100-conidia sensitivity in BAL samples [35].
We found no false-positive PCR in patients without IPA documented. This excellent specificity agrees with the most recent PCR studies on BAL [16, 21, 26] and could result from the development of real-time PCR assays that have decreased the risk of contamination [36]. Furthermore, performing PCR in BAL from patients at risk for IPA, and considering a positive PCR result only in patients with clinical findings suggesting IPA, could avoid false-positive results in colonised patients [23].
PCR and GM both reached a 88% sensitivity in BAL positive for DE/culture, which confirmed the recent studies reporting a 75–96% and 67–78% sensitivity, respectively [20, 22, 26]. The absence of DNA amplification or GM detection whereas DE/culture is positive in two patients (patients nos. 5 and 6, Table 1) confirmed previous data: false-negatives can occur but are rare [20]. Since no inhibition has been found, it could result from either the absence of fungal material in the part of BAL used for analysis or from a low yield of DNA extraction. In terms of quantification, culture and DNA detection seem not to be correlated, since the absence or very low fungal DNA quantities have been observed, whereas culture was positive (e.g. patients nos. 5 and 6, Table 1). The absence of GM detection whereas DE/culture is positive could also result from the low quantities which we failed to detect because of a too high cut-off value (≥1). Although no cut-off value has been validated in the BAL, and using lower cut-off values (e.g. 0.5 or 0.7) could have improved the performance of the assay [26], it could not be assessed here, since only qualitative results of GM were available.
In DE/culture-negative BAL, the PCR and GM sensitivity were lower (38% and 58%) and confirmed previous studies which reported a 33–36% and 41–67% sensitivity, respectively [20, 22, 26]. Positive control amplification in all samples excluded the presence of PCR inhibitors, which was consistent with previously reported increased DNA quality provided by using commercial kits [31]. The sensitivity could also have been decreased by potential damage on DNA samples (stored at −20°C and retrospectively collected) or by non-Aspergillus infections that could yield positive DE or GM (cross-reactivity with other species), whereas PCR detection remained negative.
The comparison of PCR sensitivity according to GM results yielded a lower sensitivity in GM-negative (36%) than in GM-positive (57%) BAL. Although other studies usually used GM as a criterion for the selection of patients and rarely included patients at risk of IPA with GM-negative BAL samples, our results seemed to be consistent with the recent study which included two GM-negative BALs and reported a PCR sensitivity of 50%. whereas the sensitivity of PCR in GM-positive samples reached 60% [20]. In addition, PCR was the only positive test in 3 out of 4 GM-negative–PCR-positive BAL. In these samples, PCR improved BAL sensitivity and confirmed that this highly specific test could contribute to IPA diagnosis.
Influence of antifungal treatment on the performance of Aspergillus tests
The duration of the antifungal therapy was not significantly related to the results of DE/culture, GM or PCR in our study. However, the antifungal treatment most patients received could have contributed to decrease Aspergillus tests’ sensitivity, as previously reported in experimental models of IPA [37, 38]. The absence of statistical significance could result from clinical bias, such as the low number of patients or antifungal resistance of the strain responsible for IPA.
Alveolar micro-environment: evaluation of BAL quality and influence on Aspergillus detection
Among the criteria analysed, β-globin seems to be the most discriminating, significantly related to an increased Aspergillus detection by DE/culture or PCR, suggesting that a high cellularity (reflected by a high β-globin level) could increase conidia, hypha and DNA detection. On the other hand, GM detection seems to be independent from β-globin levels, even if false-positive GM results have been described in BAL [39] and cannot be totally excluded in our study. The absence of the influence of β-globin on GM detection in BAL supernatants could also result from its release in the alveolar micro-environment during growth [7], reducing the impact of cell-recovering efficiency during bronchoscopy on its detection. This could make the GM test fairly independent of BAL quality and explain its high sensitivity compared to DE/culture and PCR, which is more dependent on BAL quality.
Detection in the cell pellet of conidia, hyphae (by DE/culture) and fungal DNA (by PCR) results from their presumed localisation in phagocytes. As suggested by previous studies using cell pellets to detect DNA [15–20, 29] and by a recent study comparing the detection of fungal DNA in BAL pellets and supernatants [33], the major localisation of fungal DNA is probably conidia and hypha, in BAL pellets, rather than free in BAL supernatants, released by live and dead fungal material. However, using BAL supernatants could contribute to IPA diagnosis and should be further evaluated in prospective studies that might contribute to improve our understanding of the physiopathological mechanisms of Aspergillus infection.
Even if cellularity followed the same trend as β-globin quantification (higher in PCR-positive and in GM-negative subgroups), there was no significant difference between subgroups when volume and cellularity were analysed (Table 3). Therefore, only β-globin quantification was found to be a significant relevant marker of BAL overall quality when we evaluated the IPA diagnosis tools. The accuracy of β-globin quantification could result from both biological and technical criteria, including cellularity, centrifugation of BAL, efficiency of DNA extraction, PCR method and the absence of inhibitors. Contrarily to cellularity, β-globin levels could be less influenced by cell lysis during the handling of the BAL fluids, whereas nuclear DNA remains in the pellet and seems more reliable to control the yield of the BAL.
The significant differences observed in β-globin subgroups could be useful for the interpretation of both DE/culture and PCR results. Given that a high level of β-globin is associated with positive PCR results in IPA patients, negative PCR with high β-globin levels could more likely reflect the true absence of Aspergillus DNA in the sample, rather than a lack of sensitivity. Similarly, when human DNA content is low, a negative Aspergillus PCR result should be interpreted carefully, since false-negatives could occur more frequently, as suggested by a recent study [33]. In BAL with high β-globin levels, the inhibition of the detection of Aspergillus DNA present in small quantities by human background DNA should also be taken into account (e.g. patients nos. 15 or 23, Table 1) [40].
Conclusion
Polymerase chain reaction (PCR) on bronchoalveolar lavage (BAL) fluids could contribute to the diagnosis of invasive pulmonary aspergillosis (IPA). Despite recent developments of real-time PCR assays resulting in a high analytical sensitivity of fungal DNA detection, the sensitivity of PCR is still lower than galactomannan (GM) detection in the BAL. Taking into account the DNA content of BAL fluids, using human β-globin DNA quantification for the interpretation of PCR results could improve the value of this test. A minimal β-globin value yielding a 100% NPV could be determined in further prospective studies, and might contribute to validate a consensus approach of fungal PCR in IPA diagnosis.
References
Pagano L, Girmenia C, Mele L, Ricci P, Tosti ME, Nosari A et al (2001) Infections caused by filamentous fungi in patients with hematologic malignancies. A report of 391 cases by GIMEMA Infection Program. Haematologica 86(8):862–870
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F et al (2002) Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 34(1):7–14. doi:10.1086/323335
Hope WW, Walsh TJ, Denning DW (2005) Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis 5(10):609–622. doi:10.1016/S1473-3099(05)70238-3
Verdaguer V, Walsh TJ, Hope W, Cortez KJ (2007) Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Rev Mol Diagn 7(1):21–32. doi:10.1586/14737159.7.1.21
Tarrand JJ, Lichterfeld M, Warraich I, Luna M, Han XY, May GS et al (2003) Diagnosis of invasive septate mold infections. A correlation of microbiological culture and histologic or cytologic examination. Am J Clin Pathol 119(6):854–858. doi:10.1309/EXBVYAUPENBM285Y
Aquino VR, Goldani LZ, Pasqualotto AC (2007) Update on the contribution of galactomannan for the diagnosis of invasive aspergillosis. Mycopathologia 163(4):191–202. doi:10.1007/s11046-007-9010-2
Mennink-Kersten MA, Donnelly JP, Verweij PE (2004) Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis 4(6):349–357. doi:10.1016/S1473-3099(04)01045-X
Tanriover MD, Metan G, Altun B, Hascelik G, Uzun O (2005) False positivity for Aspergillus antigenemia related to the administration of piperacillin/tazobactam. Eur J Intern Med 16(7):489–491. doi:10.1016/j.ejim.2005.04.007
Dalle F, Charles PE, Blanc K, Caillot D, Chavanet P, Dromer F et al (2005) Cryptococcus neoformans Galactoxylomannan contains an epitope(s) that is cross-reactive with Aspergillus Galactomannan. J Clin Microbiol 43(6):2929–2931. doi:10.1128/JCM.43.6.2929-2931.2005
Becker MJ, Lugtenburg EJ, Cornelissen JJ, Van Der Schee C, Hoogsteden HC, De Marie S (2003) Galactomannan detection in computerized tomography-based broncho-alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br J Haematol 121(3):448–457. doi:10.1046/j.1365-2141.2003.04308.x
Costa C, Costa JM, Desterke C, Botterel F, Cordonnier C, Bretagne S (2002) Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J Clin Microbiol 40(6):2224–2227. doi:10.1128/JCM.40.6.2224-2227.2002
Einsele H, Hebart H, Roller G, Löffler J, Rothenhofer I, Müller CA et al (1997) Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol 35(6):1353–1360
Ferrer C, Colom F, Frasés S, Mulet E, Abad JL, Alió JL (2001) Detection and identification of fungal pathogens by PCR and by ITS2 and 5.8S ribosomal DNA typing in ocular infections. J Clin Microbiol 39(8):2873–2879. doi:10.1128/JCM.39.8.2873-2879.2001
Halliday C, Wu QX, James G, Sorrell T (2005) Development of a nested qualitative real-time PCR assay to detect Aspergillus species DNA in clinical specimens. J Clin Microbiol 43(10):5366–5368. doi:10.1128/JCM.43.10.5366-5368.2005
Skladny H, Buchheidt D, Baust C, Krieg-Schneider F, Seifarth W, Leib-Mösch C et al (1999) Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. J Clin Microbiol 37(12):3865–3871
Spiess B, Buchheidt D, Baust C, Skladny H, Seifarth W, Zeilfelder U et al (2003) Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNA in clinical samples from neutropenic patients. J Clin Microbiol 41(5):1811–1818. doi:10.1128/JCM.41.5.1811-1818.2003
Bretagne S, Costa JM, Marmorat-Khuong A, Poron F, Cordonnier C, Vidaud M et al (1995) Detection of Aspergillus species DNA in bronchoalveolar lavage samples by competitive PCR. J Clin Microbiol 33(5):1164–1168
Buchheidt D, Baust C, Skladny H, Ritter J, Suedhoff T, Baldus M et al (2001) Detection of Aspergillus species in blood and bronchoalveolar lavage samples from immunocompromised patients by means of 2-step polymerase chain reaction: clinical results. Clin Infect Dis 33(4):428–435. doi:10.1086/321887
Raad I, Hanna H, Huaringa A, Sumoza D, Hachem R, Albitar M (2002) Diagnosis of invasive pulmonary aspergillosis using polymerase chain reaction-based detection of Aspergillus in BAL. Chest 121(4):1171–1176. doi:10.1378/chest.121.4.1171
Rantakokko-Jalava K, Laaksonen S, Issakainen J, Vauras J, Nikoskelainen J, Viljanen MK et al (2003) Semiquantitative detection by real-time PCR of Aspergillus fumigatus in bronchoalveolar lavage fluids and tissue biopsy specimens from patients with invasive aspergillosis. J Clin Microbiol 41(9):4304–4311. doi:10.1128/JCM.41.9.4304-4311.2003
Sanguinetti M, Posteraro B, Pagano L, Pagliari G, Fianchi L, Mele L et al (2003) Comparison of real-time PCR, conventional PCR, and galactomannan antigen detection by enzyme-linked immunosorbent assay using bronchoalveolar lavage fluid samples from hematology patients for diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol 41(8):3922–3925. doi:10.1128/JCM.41.8.3922-3925.2003
Verweij PE, Latgé JP, Rijs AJ, Melchers WJ, De Pauw BE, Hoogkamp-Korstanje JA et al (1995) Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. J Clin Microbiol 33(12):3150–3153
Tuon FF (2007) A systematic literature review on the diagnosis of invasive aspergillosis using polymerase chain reaction (PCR) from bronchoalveolar lavage clinical samples. Rev Iberoam Micol 24(2):89–94
Papanicolaou Society of Cytopathology Task Force on Standard Practice (1999) Guidelines of the papanicolaou society of cytopathology for the examination of cytologic specimens obtained from the respiratory tract. Diagn Cytopathol 21(1):61–69. doi:10.1002/(SICI)1097-0339(199907)21:1<61::AID-DC17>3.0.CO;2-O
Chamberlain DW, Braude AC, Rebuck AS (1987) A critical evaluation of bronchoalveolar lavage. Criteria for identifying unsatisfactory specimens. Acta Cytol 31(5):599–605
Musher B, Fredricks D, Leisenring W, Balajee SA, Smith C, Marr KA (2004) Aspergillus galactomannan enzyme immunoassay and quantitative PCR for diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. J Clin Microbiol 42(12):5517–5522. doi:10.1128/JCM.42.12.5517-5522.2004
Durand-Joly I, Chabé M, Soula F, Delhaes L, Camus D, Dei-Cas E (2005) Molecular diagnosis of Pneumocystis pneumonia. FEMS Immunol Med Microbiol 45(3):405–410. doi:10.1016/j.femsim.2005.06.006
Bolehovska R, Pliskova L, Buchta V, Cerman J, Hamal P (2006) Detection of Aspergillus spp. in biological samples by real-time PCR. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150(2):245–248
Millon L, Piarroux R, Deconinck E, Bulabois CE, Grenouillet F, Rohrlich P et al (2005) Use of real-time PCR to process the first galactomannan-positive serum sample in diagnosing invasive aspergillosis. J Clin Microbiol 43(10):5097–5101. doi:10.1128/JCM.43.10.5097-5101.2005
Martín Juan J, Valenzuela Mateos F, Soto Campos G, Segado Soriano A, Rodríguez Panadero F, Castillo Gómez J (1996) Quality and selection of samples of bronchoalveolar lavage (BAL) in diffuse pneumopathies. Arch Bronconeumol 32(7):332–340
Griffiths LJ, Anyim M, Doffman SR, Wilks M, Millar MR, Agrawal SG (2006) Comparison of DNA extraction methods for Aspergillus fumigatus using real-time PCR. J Med Microbiol 55(Pt 9):1187–1191. doi:10.1099/jmm.0.46510-0
Löffler J, Hebart H, Schumacher U, Reitze H, Einsele H (1997) Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J Clin Microbiol 35(12):3311–3312
Khot PD, Ko DL, Hackman RC, Fredricks DN (2008) Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis 8(1):73. doi:10.1186/1471-2334-8-73
Löffler J, Henke N, Hebart H, Schmidt D, Hagmeyer L, Schumacher U et al (2000) Quantification of fungal DNA by using fluorescence resonance energy transfer and the light cycler system. J Clin Microbiol 38(2):586–590
Francesconi A, Kasai M, Harrington SM, Beveridge MG, Petraitiene R, Petraitis V et al (2008) Automated and manual methods of DNA extraction for Aspergillus fumigatus and Rhizopus oryzae analyzed by quantitative real-time PCR. J Clin Microbiol 46(6):1978–1984
Bretagne S, Costa JM (2005) Towards a molecular diagnosis of invasive aspergillosis and disseminated candidosis. FEMS Immunol Med Microbiol 45(3):361–368. doi:10.1016/j.femsim.2005.05.012
Francesconi A, Kasai M, Petraitiene R, Petraitis V, Kelaher AM, Schaufele R et al (2006) Characterization and comparison of galactomannan enzyme immunoassay and quantitative real-time PCR assay for detection of Aspergillus fumigatus in bronchoalveolar lavage fluid from experimental invasive pulmonary aspergillosis. J Clin Microbiol 44(7):2475–2480. doi:10.1128/JCM.02693-05
Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW et al (2008) Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob Agents Chemother 52(7):2593–2598. doi:10.1128/AAC.00276-08
Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S et al (2008) Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med 177(1):27–34. doi:10.1164/rccm.200704-606OC
Lachnik J, Ackermann B, Bohrssen A, Maass S, Diephaus C, Puncken A et al (2002) Rapid-cycle PCR and fluorimetry for detection of mycobacteria. J Clin Microbiol 40(9):3364–3373. doi:doi:10.1128/JCM.40.9.3364-3373.2002
Acknowledgements
This work was supported by grants from the Lille 2 University Hospital of Lille and the Faculty of Medicine (Lille 2 University), and was developed in the framework of the EA 3609 scientific project (French Research Office). It was presented at the 3rd Trends in Medical Mycology (TIMM 2007), Turin, Italy, October 2007. We thank the members of the Department of Parasitology-Mycology of Lille 2 University Hospital: Fabienne Soula for her valuable help in the clinical data collection; Michèle Wauquier and Filoména Naji for their technical assistance; Boualem Sendid, Nadine François, Stéphanie Delbart, Pascale Daelman and Laurence Dumortier for their involvement in the routine mycological tests. We are grateful to Anthony Pinon (research assistant, Lille Pasteur Institute, France) for the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fréalle, E., Decrucq, K., Botterel, F. et al. Diagnosis of invasive aspergillosis using bronchoalveolar lavage in haematology patients: influence of bronchoalveolar lavage human DNA content on real-time PCR performance. Eur J Clin Microbiol Infect Dis 28, 223–232 (2009). https://doi.org/10.1007/s10096-008-0616-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0616-1