Abstract
Meropenem is a carbapenem antibiotic that is highly active against the pathogens causing meningitis. Results with meropenem in the experimental rabbit model of penumococcal meningitis have been controversial, and the possible role of renal dehydropeptidase I in meropenem efficacy has been suggested. The aim of this study was to determine the efficacy of meropenem in two meningitis models and the possible influence of the animal model over results. Two strains of Streptococcus pneumoniae with different susceptibility to beta-lactams have been used in a guinea pig model and the classical rabbit meningitis model. Meropenem was bactericidal at 6 h in the guinea pig model against both strains with a reduction of >4 log ufc/ml. In the rabbit model it was bactericidal at 6 h against the susceptible strain, but against the resistant 3/8 therapeutical failures were recorded at 6 h, being bactericidal at 24 h. In conclusion, meropenem has shown bactericidal activity in both experimental models. This work emphasises the importance of an adequate election of the animal model for the appropriate development of studies of antimicrobial efficacy. We believe that guinea pig should be considered the best choice among laboratory animal species when assessing meropenem efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite effective antibiotic therapy, pneumococcal meningitis is still associated with high rates of mortality and permanent sequel. The increasing spread of cephalosporin-resistant strains has made the therapy for pneumococcal meningitis more difficult.
Meropenem is a broad-spectrum carbapenem antibiotic that is highly active against the major bacterial pathogens causing meningitis and penetrates well into the CSF [1]. The limited experience in patients with bacterial meningitis suggests that the use of meropenem may lead to clinical and microbiologic outcomes similar to those of cefotaxime or ceftriaxone, but the number of resistant cases is very small [2–5]. Carbapenems such as imipenem or meropenem demonstrate high activity against pneumococci with different susceptibility patterns to β-lactams [6]. In spite of its high in vitro activity, imipenem is not recommended in the management of bacterial meningitis due to its convulsive potential [7]. Overall, meropenem that is not associated with this collateral effect might represent a good alternative in cephalosporin-resistant pneumococcal meningitis.
Results with meropenem in the experimental rabbit model of penumococcal meningitis have been controversial [8, 9], and the possible role of renal dehydropeptidase I (DHP-1) in meropenem efficacy has been suggested. The activity of DHP-1 differs among mammal species: meropenem is relatively stable to the human enzyme, but varies in its stability in many common laboratory animal species [10]. The drug is easily hydrolyzed by rabbits, while it shows greater resistance in guinea pigs. Both animal models have been used in order to assess the activity of meropenem in experimental meningitis [8, 11].
The aim of this study was to determine the efficacy of meropenem against two S. pneumoniae strains with different susceptibility to β-lactams using the rabbit and the guinea pig meningitis models and the possible influence of the animal model over results.
Materials and methods
Bacterial strains
Two strains of S. pneumoniae isolated from patients with meningitis were used: strain HUB SIII belonging to serotype 3 and HUB 2349 belonging to serotype 23F. MICs and MBCs were determined by the macrodilution method according to CLSI (formerly NCCLS) guidelines [12]. MICs/MBCs (in µg/ml) of strain HUB SIII were as follows: penicillin 0.01/0.01 and ceftriaxone 0.01/0.01; meropenem 0.01/0.01 MICs/MBCs (in µg/ml) of strain HUB 2349 were as follows: penicillin 4/4, ceftriaxone 2/4 and meropenem 0.5/1.
In vitro studies
Time-kill curves were performed with glass tubes containing a final volume of 10 ml of cation-adjusted Mueller-Hinton broth with 5% of horse lysed blood. The final inoculum was 5×105 CFU/ml. For each strain, meropenem was tested for a range of concentrations according to their MICs and their achievable concentration in CSF. Meropenem was tested at concentrations ranging from 1/4 to 32 × MIC. Samples were removed at 0, 6 and 24 h of incubation. The detection limit was 10 CFU/ml. Bactericidal effect was defined as a decrease of ≥3 log CFU/ml of the initial inoculum.
Guinea pig model
The study was approved by the Ethical Committee for Animal Experiments at the University of Barcelona. The meningitis model described by Maiques et al. [13] based in a wide modification of that of Nairn [11] was used. For all experiments, guinea pigs were challenged in groups of at least eight animals. Hartley female guinea pigs weighing 350–450 g were anaesthetized by a single im injection of a mixture of xilacine: 4 mg/kg (Rompum; Bayer AG, Leverkusen, Germany) and ketamine: 30 mg/kg (Ketolar; Parke-Davis, Prat de Ll., Spain). Without the use of any stereotactic system, a 27-gauge butterfly needle with infusion set was inserted, through the nucal area, into the cisterna magna. When clear CSF flowed, 100 µl of saline solution containing 106 CFU/ml of one of the infecting strains was injected intrathecally, and the butterfly removed. Eighteen hours after inoculation the animals were anaesthetized again, and a baseline CSF sample was taken (0h) by the same procedure as inoculation. A blood sample was collected to assess secondary bacteraemia. Meropenem: 200 mg/kg/6 h (Meronem; AstraZeneca, Madrid, Spain) im was then administered for 24 h. Untreated controls received saline. CSF samples were taken at 3, 6 and 24 h of therapy. The available CSF volume was limited by the small size of guinea pigs, so we arranged two groups in order to have a final n≥8 samples for each time point. In one group, CSF samples were taken at 0 and 6 h, and in the other at 3 and 24 h.
Rabbit model
The rabbit model of meningitis was based on an established protocol [14]. For all experiments, rabbits were challenged in groups of at least eight animals. Two-kilogram female New Zealand white rabbits were anaesthetized im with 35 mg/kg of ketamine and 5 mg/kg of xylazine, and a dental acrylic helmet was affixed to the calvarium; 24 h later, animals were anaesthetized again and placed in a stereotaxic frame. Meningitis was induced using an intracisternal injection of 250 µl of saline solution containing 106 CFU/ml inoculum of one of the infecting strains. Eighteen hours later, rabbits were anaesthetized with urethane 1.75 g/kg, subcutaneously (Sigma Chemical Company, St. Louis, MO) and thiopental sodium 5 mg/kg, iv (Tiopental; B. Braun Medical S.A., Rubí, Spain), and a baseline CSF sample was taken (0h). A blood sample was collected to assess secondary bacteraemia. Meropenem: 150 mg/kg/6 h iv was then administered for 24 h. Untreated controls received saline. CSF samples were taken at 2, 6 and 24 h of therapy.
Sample processing
CSF samples were used to determine bacterial counts, lactate and protein concentrations, and antibiotic levels at peak and trough time points. Undiluted cultures and serial ten-fold dilutions were performed at each time point. The lowest bacterial concentration detectable was 10 CFU/ml, so a value of 0.99 log CFU/ml was assigned to sterile culture. Therapeutic failure was defined as an increase in bacterial concentration of at least 1 log CFU/ml compared with a previous count and bacterial re-growth an increase of <1 log CFU/ml. A therapy was considered bactericidal when a reduction of ≥3 log CFU/ml was achieved. Lactate concentrations were measured using a Lactate PAP (Biomérieux, France). Protein concentrations were determined using the Bradford method (Bio-Rad Protein Assay, Germany).
Pharmacokinetics
Pharmacokinetic (PK) studies were performed in both models to select dose regimens that result in CSF concentrations similar to those in humans. PK data were compiled from a study of infected guinea pig after a single i.m. dose of 200 mg/kg of meropenem and from a study of infected rabbits after a single i.v. dose of 150 mg/kg of meropenem. Several PK and pharmacodynamic (PD) parameters were determined in serum and CSF: maximum concentration (Cmax), area under the concentration-time curve (AUC), CSF penetration as the comparison of areas under the curve (AUCCSF/AUCserum) and time above the MIC of the drug concentration in CSF (t >MIC). PK and PD parameters were obtained by a computer-assisted method (PK functions for Microsoft Excel; J.I. Usansky, A. Desai and D. Tang-Liu, Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA) after determination of antibiotic concentration in blood and CSF samples at the different time points.
Antibiotic assays
Meropenem concentrations were measured by agar disc diffusion method [15], using Bacillus subtilis ATCC 12432 as assay organism.
Statistical analysis
All data were checked for normal distribution (Kolmogorov-Smirnov test). Comparisons between groups were made by using Student's t test. P<0.05 was considered statistically significant.
Results
In vitro killing curves
Strain HUB SIII
Meropenem at 1–32 × MIC (0.01–0.32 µg/ml) was bactericidal at 6 h. Strain HUB 2349. Meropenem at 2–4 x MIC (1–2 µg/ml) was bactericidal at 6 h.
Pharmacokinetics
Meropenem peak level in serum was 125.44±28.22 μg/ml at 0.30 h in guinea pigs and 71.30±24.44 μg/ml at 0.30 h in rabbits. Pharmacokinetic and pharmacodynamic parameters of antibiotics in CSF of guinea pigs and rabbits with pneumococcal meningitis are shown in Table 1.
Experimental meningitis in guinea pig
Strain HUB SIII
Eighteen hours after inoculation, 100% of the animals presented secondary bacteraemia. Mortality at 24 h was 44% (4/9) in the control group and 0% in the therapy group. Table 2 shows the bacterial reduction in CSF at 6 and 24 h; meropenem were bactericidal from 6 h. No therapeutic failures were observed. Antibiotic levels in CSF of animals infected with this strain are summarized in Table 3. After 24 h of infection, CSF inflammatory parameters were decreased in the therapy group compared to the control group, with lactate levels being statistically significant (in mmol/l: meropenem, 1.48±0.41 and control group, 4.16±0.65).
Strain HUB 2349
Eighteen hours after inoculation, 100% of the animals showed secondary bacteraemia. Mortality at 24 h was 0% in the control and therapy group. Table 2 shows the bacterial reduction in CSF at 6 and 24 h; meropenem was bactericidal from 6 h. No therapeutic failures were observed. Antibiotic levels in CSF of animals infected with this strain are represented in Table 3. Meropenem reduced CSF inflammatory parameters in comparison to the control group at 24 h, with lactate levels being statistically significant (in mmol/l: meropenem, 1.99 ± 0.33 and control group, 3.78±1.89).
Experimental meningitis in rabbits
Strain HUB SIII
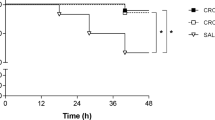
Eighteen hours after inoculation, 63% of the animals presented secondary bacteraemia. Mortality at 24 h was 63% (7/11) in the control group and 0% in the therapy group. Table 4 represents CSF bacterial counts at 0 h and CSF bacterial reduction at 2, 6 and 24 h; meropenem was bactericidal from 6 h. No therapeutic failures were observed. Antibiotic levels in the CSF of animals infected with the HUB SIII strain are summarized in Table 3. Meropenem reduced CSF inflammatory parameters in comparison to the control group at 24 h, with lactate levels being statistically significant (in mmol/l: meropenem, 1.95±0.92 and control group, 3.62±0.86).
Strain HUB 2349
Eighteen hours after the inoculation, 100% of the animals presented secondary bacteraemia. Mortality at 24 h was 62% (five of eight) in the control group and 0% in the therapy group. Initial CSF bacterial concentrations and bacterial decreases at 2, 6 and 24 h of different regimens are summarized in Table 4. After 6 h of meropenem therapy, therapeutic failure was present in three of eight animals and bacterial regrowth in the other three animals. Meropenem was bactericidal at 24 h. Antibiotic levels in the CSF of animals infected with the HUB 2349 strain are shown in Table 3. No statistical differences were noted in CSF inflammatory parameters.
Discussion
In our study, meropenem has shown great efficacy in the guinea pig model of meningitis caused by two pneumococcal strains with different susceptibility to β-lactams. With a CSF penetration of 5.8%, mean peak CSF levels closely related to those obtained in children receiving 20 mg/kg i.v were reached [1]. In efficacy experiments, no therapeutic failures were observed in any animal despite its relatively low levels, especially noted in guinea pigs infected with the penicillin-resistant pneumococcus. T>MIC was more than 100% for the SIII strain, whereas it was about 70% for the HUB 2349 strain. It seems that a value of 70% would be necessary to reach a bactericidal effect when using meropenem in the guinea pig model. The use of this laboratory animal to study meropenem efficacy has been previously reported by Nairn et al [11]. They assessed meropenem efficacy against several pathogens causing meningitis, including either penicillin-susceptible or penicillin-resistant strains of S. pneumoniae in a 10-h experiment. Meropenem at 10 mg/kg/3 h reduced bacterial counts below the level of detection in all guinea pigs infected with penicillin-susceptible strain. In animals inoculated with penicillin-resistant strain, meropenem at 40 mg/kg/3 h sterilised CSF specimens in four of five animals.
Using the rabbit meningitis model, meropenem was effective against the β−lactam susceptible strain. However, in the experiments with the resistant strain, efficacy was delayed, and at 6 h we observed therapeutic failure in three of eight animals and bacterial regrowth in the other three animals. After 24 h, meropenem was bactericidal against both strains, although statistically significant differences were noted among rabbits infected with either the susceptible or the resistant strain (rlog cfu/ml were, respectively: –4.32±0.38, and –3.36±1.25; p=0.007). CSF meropenem concentrations were similar among rabbits infected with each pneumococcal strain, although slightly lower levels were found at 6 h for the resistant strain. Therapeutic failures and regrowth occurred in the resistant-strain-infected animals. This fact suggests that drug levels very close to MIC might not be high enough to ensure efficacy despite a T>MIC above 85%. In comparison with findings in the guinea pig model, meropenem failure could be associated to the rapid hydrolysis of DHP-I rather than an unfavourable CSF kinetics. Therefore, in accordance to previous reports, we chose a dose of 150 mg/kg in order to compensate rapid enzymatic hydrolysis. However, this dose was not higher enough to ensure a bactericidal activity in all tested animals. Furthermore, we tested antimicrobial efficacy for a longer period in comparison with other studies, so this time schedule limited the interval dosing. Nevertheless, shorter intervals would not ensure higher efficacies [15]. Meropenem has been tested at 125 mg/kg in abbreviated experiments against pneumococcal strains with different results. Friedland et al. reported poor efficacy against two resistant pneumococcal strains, although CSF peak levels were eightfold greater than the MICs [9]. On the other hand, some authors have described a bactericidal effect when administering meropenem every 4 h [8, 15]. However, in one of these studies meropenem failed, and CSF sterilization was only reached in two of eight animals after 8 h of therapy [16]. Our in vitro results confirm the dose-related bactericidal activity previously reported when meropenem concentrations above the MIC are tested [8]. According to these results and our own data, we would suggest that meropenem might be a good alternative in the therapy of pneumococcal meningitis, but the classic rabbit model is not the best model to study meropenem activity, and another model without pharmacokinetic problems, such as the guinea pig model, might be better to study meropenem efficacy.
The rabbit model has been extensively used in order to assess antimicrobial activity in bacterial meningitis. The guinea pig model of experimental meningitis presents some limitations in comparison with the rabbit one. The small animal size does not allow serial CSF collection from the same animal, although we have used the same animal for two different time points. The use of this laboratory animal has been scarce in the study of alternative therapies for bacterial meningitis [13], so information about CSF kinetics is difficult to analyze. However, our results support the use of the guinea pig model as a good alternative in the study of meropenem efficacy.
In conclusion, meropenem has shown bactericidal activity in both experimental models of meningitis and might be considered as an alternative in the management of pneumococcal meningitis. On the other hand, this work emphasises the importance of an adequate election of the animal model for the appropriate development of studies of antimicrobial efficacy. We believe that guinea pig should be considered the best choice among laboratory animal species when assessing meropenem efficacy, even considering the exposed limitations.
References
Dagan R, Velghe L, Rodda JL, Klugman KP (1994) Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J Antimicrobial Chemother 34:175–179
Schmutzhard E, Williams KJ, Vukmirovits G, Chemlik V, Pfausler B, Featherstone A (1995) A randomize comparison of meropenem with cefotaxime or ceftriaxone for the treatment of bacterial meningitis in adults. Meropenem Meningitis Study Group J Antimicrobial. Chemother 36(Suppl A):85–97
Klugman KP, Dagan R, Meropenem Study Group (1995) Randomized comparison of meropenem with cefotaxime for treatment of bacterial meningitis. Antimicrob Agents Chemother 39:1140–1146
Odio CM, Puig JR, Feris JM, Khan WN, Rodriguez WJ, McCracken GH, Bradley JD (1999) Prospective, randomized, investigator-blinded study of the efficacy and safety of meropenem vs. cefotaxime therapy in bacterial meningitis in children Meropenem Study Group. Pediatr Infect Dis J 18:581–590
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, Whitley RJ (2004) Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 39:1267–1284
Fitoussi F, Doit C, Benali K, Boacorsi S, Gelsin P, Bingen E (1998) Comparative in vitro killing activities of meropenem, imipenem, ceftriaxone and ceftriaxone plus vancomycin at clinically achievable cerebrospinal fluid concentrations against penicillin resistant Streptococcus pneumoniae isolates from children with meningitis. Antimicrob Agents Chemother 42:942–944
Norrby SR (2000) Neurotoxicity of carbapenem antibiotics: consequences for their use in bacterial meningitis. J Antimicrobial Chemother 45:5–7
Gerber CM, Cottagnoud M, Neftel KA, Täuber MG, Cottagnoud P (1999) Meropenem alone and in combination with vancomicin in experimental meningitis caused by a penicillin-resistant pneumoccocal strain. Eur J Clin Microbiol Infect Dis 18:886–870
Friedland IR, Paris M, Ehrett S, Hickey S, Olsen K, McCracken JR (1993) Evaluation of antimicrobial regimens for treatment of experimental penicillin and cephalosporin resistant pneumococcal meningitis. Antimicrob Agents Chemother 37:1630–1636
Fukasawa M, Sumita Y, Harabe ET, Tanio T, Nouda H, Kohzuki et al (1992) Stability of meropenem and effect of 1β-methil substitution on its stability in the presence of renal dehydropeptidase I. J Antimicrobial Chemother 36:1577–1579
Nairn K, Shepherd GL, Edwards JR (1995) Efficacy of meropenem in experimental meningitis. J Antimicrobial Chemother 36(Suppl A):73–84
National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically-(2000) 5th edn: Approved Standard M7-A5 NCCLS, Wayne, PA
Maiques JM, Domenech A, Cabellos C, Fernández A, Ribes S, Tubau F et al (2007) Evaluation of antimicrobial regimens in a guinea-pig model of meningitis caused by Pseudomonas aeruginosa. Microb Infect 9:435–441
Dacey RG, Sande MA (1974) Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother 6:437–41
Chapin-Robertson K, Edberg SC (1991) Measurements of antibiotics in human body fluids: techniques and significance. In: Lorian V (ed) Antibiotics in laboratory medicine. Williams and Wilkins, New York, pp 295–366
Cottagnoud P, Cottagnoud M, Acosta F, Flatz L, Kühn, Stucki A, Entenza J (2003) Meropenem prevents levofloxacin induced resistance in penicillin resistant pneumococci and acts synergistically with levofloxacin in experimental meningitis. Eur J Clin Microbiol Infect Dis 22:656–662
Acknowledges
This work was supported by a research grant from the “Fondo de Investigaciones Sanitarias” FIS 02/069 from “Instituto de Salud Carlos III, Ministerio de Sanidad”, Spain. A. Domenech and F. Taberner were supported by grants from the Universitat de Barcelona and S. Ribes was supported by the same FIS grant. All authors are members of the Spanish Network for the Research in Infectious Diseases (REIPI CO3/14 and REIPI RD06/0008). All authors: No commercial support. All experiments were performed complying with current law for animal models (decret 214/1997 DOGC august 7th and RD 1201/2005 october 10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Force, E., Taberner, F., Cabellos, C. et al. Experimental study of meropenem in the therapy of cephalosporin-susceptible and -resistant pneumococcal meningitis. Eur J Clin Microbiol Infect Dis 27, 685–690 (2008). https://doi.org/10.1007/s10096-008-0492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0492-8