Abstract
The aim of this study was to measure the seroprevalence to mumps in Norwegian conscripts belonging to the first children vaccination cohorts that had been offered two doses of MMR vaccine. The seroprevalence to mumps was 76% with the Microimmune assay and 85% with the Enzygnost assay. We also compared the performance of the Microimmune assay for detection of mumps- and measles-specific IgG antibodies in 340 paired serum and oral fluid samples from the conscripts and evaluated the effect of revaccination. Mumps-specific IgG antibodies were detected in only 61% of the oral fluids. In contrast, high levels of measles-specific IgG antibodies were detected in both the serum and oral fluid samples. Based on these results, we are only able to recommend the use of oral fluid for surveillance of measles in Norway. Our results may also indicate that the seroprevalence necessary to interrupt transmission of mumps has not been reached in vaccinated young adult Norwegians. Seroconversion was observed in all initially measles seronegative conscripts after revaccination, whereas 23 of 27 initially mumps seronegative conscripts failed to seroconvert.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European Regional Office of WHO has set a target of elimination of measles and reducing the incidence of mumps to less than 1 per 100,000 by 2010. Both high vaccine coverage and national surveillance are necessary to reach these goals. Since the introduction of the combined measles, mumps, and rubella (MMR) vaccine at 15 months and at 12–13 years of age in the Norwegian National Immunization program in 1983, national coverage has been 90–95% for both doses [1]. Evaluation of immunization programmes depends on continuous examination of the seroprevalence to these vaccine-preventable diseases in the population. Standardization and evaluation of laboratory tests are particularly important in order to obtain reliable seroprevalence data; however, the full potential of antibody prevalence studies is difficult to achieve as long as such studies depend on collection of blood samples.
Mumps is an acute viral disease with parotitis as the most common clinical manifestation. Although the infection is generally mild and self-limited, serious complications such as meningoencephalitis and orchitis are not uncommon. The annual incidence of mumps in Norway has decreased to less than 10 cases per year in the last decade [2], except in 2006 (24 cases) when an outbreak struck Buskerud county between June and October (unpublished). The seroprevalence to mumps, in contrast to measles [3], is unknown in Norway.
The use of oral fluid as a noninvasive alternative to blood for detection of virus-specific IgG antibodies was initially described in 1987 [4], and several studies have shown that oral fluid samples may be adequate substitutes for serum samples for detection of both measles- and mumps-specific IgG antibodies [5–12]. Although the detection of IgG antibodies to measles and mumps in oral fluids by in-house methods has been described in several reports, only a limited number of commercial assays have been developed for antibody detection in oral fluid [9].
This is the first study presenting seroprevalence data on mumps in a vaccinated cohort of young healthy Norwegian conscripts. We also compared the detection of measles- and mumps-specific IgG antibodies in paired samples of serum and oral fluid with the commercial Microimmune assays (Microimmune, London, UK). This assay was further compared to the Enzygnost assays (Enzygnost; Dade-Behring, Berlin, Germany) for detection of IgG antibodies in serum samples. The use of military conscripts to monitor the seroprevalence in young adult Norwegians is convenient, since they may represent a randomized selection of healthy persons from all parts of the country.
This study had three goals. The first goal was to assess the seroprevalence to mumps among young adult Norwegians belonging to the first cohorts that were offered two doses of MMR vaccine in the children vaccination program. The second goal was to evaluate measles- and mumps-specific IgG detection in oral fluid, and the third goal was to assess the effect of revaccination with MMR vaccine.
Materials and methods
Study population
The study population comprised a part of the conscripts enrolled for military service in August 2004 [3]. The first 340 conscripts, who agreed to participate in that study, were included in our study. The participants were from all parts of the country. The age range was 19–27 (median 19 years). Both males and females were recruited into the study; however, less than 5% of the participants were females. The subjects belonged to the first cohorts that had been offered two doses of MMR vaccine at 15 months and at 12–13 years of age in the Norwegian children vaccination program. However, their individual MMR vaccination status was unknown. The vaccine coverage in this age group was 90–95% for two doses [1]. All participants received a booster dose of MMR vaccine after collection of samples on the day of enrolment.
Sample collection
Paired blood (S1) and oral fluid samples were collected from all 340 conscripts on the day of enrolment. A second blood sample (S2) was collected from 144 of these participants after 8 months of service. The oral fluids, which were collected using the Oracol device (Malvern Medical Developments, Worcester, UK), were extracted as described by Brown in 1994 [13]. The oral fluid extracts and serum samples were stored at –20°C prior to testing.
Analysis of measles and mumps IgG antibodies
Two different commercial ELISA assays were used for each agent: an indirect enzyme-linked immunosorbent assay (Enzygnost; Dade-Behring, Berlin, Germany) and an antibody capture immunoassay (Microimmune, London, UK). All assays were performed as recommended by the manufacturers. Samples were interpreted qualitatively as positive, negative or equivocal according to the manufacturers’ instructions. Samples with equivocal results were retested, and equivocal results after retesting were considered negative.
With the Enzygnost assay, the cut-off for qualitative evaluation of positivity was an optical density (OD) of >0.2 at 450 nm, corresponding to approximately 400 U/ml, as recommended by the manufacturer. With the Microimmune assay, the cut-off values to identify measles and mumps positivity were calculated separately for each run, according to manufacturer’s instructions.
All S1 samples were analysed for IgG antibodies against measles and mumps with both the Enzygnost and the Microimmune assays. The oral fluid samples were analysed with the Microimmune assays and the S2 samples were analysed with the Enzygnost assays. Analysis of S2 samples by the Microimmune assay was restricted to samples that tested mumps IgG negative by the Enzygnost assay.
Statistical analyses
The percentages of samples with positive and negative results were determined for each assay. The groups were compared using the chi-square test. P values of <0.05 were considered statistically significant. Differences in antibody levels between pre- and postvaccination sera were compared with Wilcoxon signed-rank order correlation test or paired t test, and correlations were assessed by the Spearman rank order correlation test applying the SigmaStat program from Software Inc. (Richmond, CA, USA). P<0.05 was considered significant.
Results
Detection of mumps-specific IgG antibodies in serum and oral fluid samples
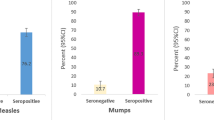
The results from detection of mumps-specific IgG antibodies in paired serum (S1) and oral fluid samples are presented in Tables 1 and 2. As shown in Table 1, 76% (95% CI: 72–81%) of the serum samples and 61% (95% CI: 56–66%) of the oral fluids were IgG positive with the Microimmune assay. Significantly higher prevalence of IgG antibodies was detected in serum samples compared to oral fluids (Chi-square test; P < 0.0001). As shown in Table 2, detection of IgG antibodies in sera increased to 85% (95% CI: 82–89%) with the Enzygnost assay. Significantly more IgG positive sera were detected with the Enzygnost assay compared to the Microimmune assay (Chi-square test; P = 0.0026). A high and significant correlation was seen between the Enzygnost and the Microimmune assays (Spearman rank order correlation coefficient = 0.774; P = 0.0001).
Detection of measles-specific IgG antibodies in serum and oral fluid samples
Measles-specific IgG antibodies in paired serum (S1) and oral fluid samples are presented in Tables 3 and 4. As shown in Table 3, IgG antibodies were detected in 96% (95% CI: 93–98%) of the serum samples and in 95% (95% CI: 93–97%) of the oral fluids with the Microimmune assay. Discordant IgG results were only obtained in 10 samples. As shown in Table 4, significantly more IgG positive sera were detected with the Microimmune assays (96%) compared to the Enzygnost assay (89%) (Chi-square test; P = 0.0007). The correlation between the Enzygnost and the Microimmune assays for measles IgG was highly significant (Spearman rank order correlation coefficient = 0.743; P = 0.0001) (Table 4).
Detection of measles and mumps IgG antibodies 8 months after revaccination
A second serum sample (S2) was obtained from 144 conscripts of whom 20 and 27 were measles and mumps IgG negative in the corresponding S1 sample by the Enzygnost assay, respectively. Mumps-specific IgG antibodies were only detected in four of the 27 S2 samples, whereas all the S2 samples were measles IgG positive. With the Microimmune assay, eight more S2 samples were mumps IgG positive. The serum IgG level against measles, measured by mean optical density (OD), increased significantly from 0.48 in S1 to 0.64 in S2 8 months after vaccination (Wilcoxon signed-rank test; P = < 0.001). The serum IgG level against mumps decreased from mean OD value 0.52 in S1 to 0.42 in S2.
Discussion
The Enzygnost assay gave a significantly higher proportion of mumps IgG positive results and a higher proportion of equivocal results than the Microimmune assay. The majority of the study population, who belonged to the first cohorts that had been offered two doses of MMR vaccine, had most likely received at least one dose of MMR vaccine due to the high vaccine coverage for this age group in Norway. The mumps IgG positivity obtained in this study is slightly lower than expected, since the seroconversion rate following mumps vaccination has been between 80% and 100% [14–16]. The discordance between vaccine coverage and seroprevalence to mumps may partly be due to a low sensitivity of the detection methods used, lack of method standardization, or low response to mumps vaccination. The seroprevalence obtained in this study is, however, in concordance with seroprevalences obtained in similar study population in other studies (76–96%) [5, 14, 16–20]. In contrast to our results, Backhouse et al. [5] demonstrated that the Microimmune assay detected significantly more IgG positive serum samples (93%) than the Enzygnost assay (54%) in a vaccinated population 17–19 years of age. The difference between our findings is surprising. The seroprevalence to mumps in our study population is slightly below the level of 90–92% that is required to achieve herd immunity [16, 18]. No mumps outbreaks have been observed in Norway in the age group studied; however, a local outbreak of mumps struck Buskerud county in 2006 (unpublished).
The present study was part of a larger study comprising 1,405 conscripts, and an equally high seroprevalence to measles was obtained in both studies [3]. The seroprevalence to mumps obtained in this study with the smaller population may therefore reflect the seroprevalence to mumps in vaccinated young adults in Norway. Males constituted the majority of the study population; however, no significant differences have been demonstrated between seroprevalence and gender [18, 20]. A relatively large proportion of sera may contain low levels of mumps-specific IgG antibodies in vaccinated individuals [21]. Low positive sera may therefore be missed when equivocal samples are interpreted as negative. The proportion of IgG positive sera increased to 90% with the Enzygnost assay and to 79% with the Microimmune assay when equivocal results were interpreted as positive (data not shown). These results may indicate that when equivocal samples in vaccinated individuals are interpreted as negative, the sera containing low levels of mumps IgG antibodies are missed and the seroprevalence is underestimated in the population.
A significantly lower proportion of oral fluids were mumps-specific IgG positive compared to the paired serum samples. A low detection of mumps IgG in oral fluids has been reported earlier [11]. It is well known that the antibody concentration in oral fluid is significantly lower than in serum, and lack of sensitivity in oral fluid-based assays has been a problem [22]. To our knowledge only a few studies have evaluated the detection of mumps IgG antibodies in oral fluids [5, 6, 11, 12]. The proportion of IgG positive oral fluids increased to only 70% when equivocal results were interpreted as positive. The overall low proportion of mumps IgG positive samples, both serum and oral fluids, may be due to a low sensitivity of the Microimmune assay.
Due to the low detection of mumps-specific IgG antibodies in oral fluids with the Microimmune assay, we do not recommend the use of oral fluid for serosurveillance of mumps in vaccinated young adults. In contrast, a high and concordant measles-specific IgG positivity was obtained in the paired serum and oral fluid samples. In concordance with other studies, our results indicate that oral fluid may replace serum in serosurveillance of measles [10, 23, 24].
In contrast to mumps, a good concordance was seen between measles IgG results using serum and oral fluid. A likely explanation may be that the Microimmune assay has a higher sensitivity for detecting measles compared to mumps. However, it may also partly be due to a stronger antibody response to the measles component of the vaccine.
The present study also demonstrated the effect of an additional dose of MMR vaccine on seroprevalence in young adult Norwegians. Whereas all the initially measles seronegative conscripts seroconverted after revaccination, 23 initially mumps seronegative conscripts failed to seroconvert. As their vaccination history is unknown, it is difficult to conclude whether there has been a primary or secondary vaccine failure. Surprisingly, a lower mean OD value for mumps IgG antibodies was measured in S2 samples compared to S1 samples. The low seroconversion rate and the reduction in the mean OD value for mumps IgG antibodies after revaccination may be due to methodological difficulties. In our laboratory, we have experienced a great variation in detection of mumps-specific IgG antibodies with different methods (unpublished data). This is especially the case when analysing samples with OD values near the cut-off value.
To reach the WHO goals for measles elimination and mumps reduction by 2010, it is important for European countries to sustain high and efficient MMR vaccination coverage. It is therefore important to evaluate the immunization programmes by monitoring the level of vaccine-induced antibodies in the population, especially in a period of decreasing opportunity for natural boosting. Detection of antibodies in oral fluids would make seroepidemiological surveillance easier; however, further improvement and standardization of commercial oral fluid-based assays are needed.
References
Anonymous (2002) Measles and measles immunization in Norway; historical review and present situation. Eurosurveillance 6
MSIS (2007) Norwegian surveillance system for communicable diseases. http://www.msis.no/. Cited 17 September 2007
Vainio K, Samdal HH, Anestad G, Skutlaberg DH, Bransdal KT, Mundal R, Aaberge I (2007) Seroprevalence of measles among Norwegian military conscripts in 2004. Eur J Clin Microbiol Infect Dis 26:217–220
Parry JV, Perry KR, Mortimer PP (1987) Sensitive assays for viral antibodies in saliva: an alternative to tests on serum. Lancet 2:72–75
Backhouse JL, Gidding HF, McIntyre PB, Gilbert GL (2006) Evaluation of two enzyme immunoassays for detection of immunoglobulin G antibodies to mumps virus. Clin Vaccine Immunol 13:764–767
Garrido RM, Blanco QA, Garrote Adrados JA, Telleria Orriols JJ, Arranz SE (1997) Value of salivary antibodies for determining seropositivity to measles, rubella, and mumps in children and adults. An Esp Pediatr 47:499–504
Gill J, Aston R, Vyse AJ, White JM, Greenwood A (2002) Susceptibility of young offenders to measles and rubella: an antibody prevalence study using oral fluid samples. Commun Dis Public Health 5:314–317
Kremer JR, Muller CP (2005) Evaluation of commercial assay detecting specific immunoglobulin G in oral fluid for determining measles immunity in vaccinees. Clin Diagn Lab Immunol 12:668–670
Nigatu W, Nokes DJ, Enquselassie F, Brown DW, Cohen BJ, Vyse AJ, Cutts FT (1999) Detection of measles specific IgG in oral fluid using an FITC/anti-FITC IgG capture enzyme linked immunosorbent assay (GACELISA). J Virol Methods 83:135–144
Nokes DJ, Enquselassie F, Nigatu W, Vyse AJ, Cohen BJ, Brown DW, Cutts FT (2001) Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull World Health Organ 79:588–595
Perry KR, Brown DW, Parry JV, Panday S, Pipkin C, Richards A (1993) Detection of measles, mumps, and rubella antibodies in saliva using antibody capture radioimmunoassay. J Med Virol 40:235–240
Thieme T, Piacentini S, Davidson S, Steingart K (1994) Determination of measles, mumps, and rubella immunization status using oral fluid samples. JAMA 272:219–221
Brown DW, Ramsay ME, Richards AF, Miller E (1994) Salivary diagnosis of measles: a study of notified cases in the United Kingdom, 1991–3. BMJ 308:1015–1017
Amela C, Pachon I, de Ory F (2003) Evaluation of the measles, mumps and rubella immunisation programme in Spain by using a sero-epidemiological survey. Eur J Epidemiol 18:71–79
Muhlemann K (2004) The molecular epidemiology of mumps virus. Infect Genet Evol 4:215–219
Nardone A, Pebody RG, van den Hof S, Levy-Bruhl D, Plesner AM, Rota MC, Tischer A, Andrews N, Berbers G, Crovari P, Edmunds WJ, Gabutti G, Saliou P, Miller E (2003) Sero-epidemiology of mumps in western Europe. Epidemiol Infect 131:691–701
Dominguez A, Plans P, Costa J, Torner N, Cardenosa N, Batalla J, Plasencia A, Salleras L (2006) Seroprevalence of measles, rubella, and mumps antibodies in Catalonia, Spain: results of a cross-sectional study. Eur J Clin Microbiol Infect Dis 25:310–317
Huerta M, Davidovitch N, Aboudy Y, Ankol OE, Balicer RD, Zarka S, Grotto I (2006) Declining population immunity to mumps among Israeli military recruits. Vaccine 24:6300–6303
Janaszek-Seydlitz W, Bucholc B, Gorska P, Slusarczyk J (2005) Mumps in Poland since 1990 to 2003; epidemiology and antibody prevalence. Vaccine 23:2711–2716
Mossong J, Putz L, Schneider F (2004) Seroprevalence of measles, mumps and rubella antibodies in Luxembourg: results from a national cross-sectional study. Epidemiol Infect 132:11–18
Vyse AJ, Gay NJ, Hesketh LM, Pebody R, Morgan-Capner P, Miller E (2006) Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol Infect 134:1303–1312
Gay NJ, Vyse AJ, Enquselassie F, Nigatu W, Nokes DJ (2003) Improving sensitivity of oral fluid testing in IgG prevalence studies: application of mixture models to a rubella antibody survey. Epidemiol Infect 130:285–291
Madar R, Straka S, Baska T (2002) Detection of antibodies in saliva—an effective auxiliary method in surveillance of infectious diseases. Bratisl Lek Listy 103:38–41
Nigatu W, Nokes DJ, Afework A, Brown DW, Cutts FT, Jin L (2006) Serological and molecular epidemiology of measles virus outbreaks reported in Ethiopia during 2000–2004. J Med Virol 78:1648–1655
Acknowledgments
This study was supported by the Norwegian Institute of Public Health and by the Norwegian Armed Forces. We thank H. Fremstad, W. Holmen, and A. Lund for excellent technical assistance. We also thank the remaining staff of our laboratory, I. J. Rodal and the military staff who participated in sample collection during the registration for primary military service. All experiments in this project comply with the current laws in Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vainio, K., Samdal, H.H., Ånestad, G. et al. Detection of measles- and mumps-specific IgG antibodies in paired serum and oral fluid samples from Norwegian conscripts. Eur J Clin Microbiol Infect Dis 27, 461–465 (2008). https://doi.org/10.1007/s10096-008-0460-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-008-0460-3