Abstract
The parasite Trichomonas vaginalis causes one of the most common non-viral sexually transmitted infections in humans. Mycoplasmas are frequently found with trichomonads but the consequences of this association are not yet known. In the present study, the effects of T. vaginalis harboring M. hominis on human vaginal epithelial cells and on MDCK cells are described. The results were analyzed by light, scanning and transmission electron microscopy, as well as using cell viability assays. There was an increase in the cytopathic effects on the epithelial cells infected with T. vaginalis associated with M. hominis compared to T. vaginalis alone. The epithelial cells exhibited an increase in the intercellular spaces, a lesser viability, and increased destruction provoked by the infected T. vaginalis. In addition, the trichomonads presented a higher amoeboid transformation rate and an intense phagocytic activity, characteristics of higher virulence behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichomonas vaginalis is a common cause of vaginitis, with worldwide distribution. Trichomoniasis is associated with damage to superficial vaginal epithelium and a profuse, acute inflammatory discharge [1, 2]. The parasite infects the urogenital tract in humans displaying high tissue and host specificity, and is responsible for the most prevalent non-viral sexually transmitted disease [3]. Trichomoniasis has been correlated with enhanced susceptibility to HIV transmission [4, 5] and possibly to cervical neoplasia [6], but the mechanisms of infection and association with other microorganisms remain poorly understood.
The association of mycoplasmas with T. vaginalis has been described previously. Mycoplasma hominis was first reported in T. vaginalis in 1975 [7], and one group proposed that these bacteria could remain intact in the trichomonad cytoplasm [8]. In recent years, there has been strong evidence showing that numerous pathogenic mycoplasmas can be intracellularly located, which allows bacterial survival over extended periods [9–13]. Also, a probably symbiotic relationship between these two organisms has been suggested [12, 13]. However, the influence of this co-infection on trichomonad behavior or its cytopathogenicity has not yet been determined. In addition, it has recently been shown that the symbiosis of M. hominis in T. vaginalis may be linked to drug resistance [14].
In this report, our group searched for consequences in the host cells and in trichomonads behavior when T. vaginalis are mycoplasma-infected. Firstly, a non-transformed human vaginal epithelial cell culture was established. Other cell cultures were also used, such as MDCK. Next, cytopathic effects provoked by T. vaginalis infected or non-infected by M. hominis were analyzed.
Materials and methods
Cell culture
Epithelial cells
HVECs
Small fragments of vaginal mucosa were obtained during a benign gynecological surgery, from a healthy post-menopause donor with informed consent. The fragments (ca. 0.5 mm) were incubated in KM/F12 (keratinocytes medium/Dulbecco modified Eagle medium) at 37°C in an atmosphere of 5% CO2. The cell confluence was reached usually within 1–2 weeks. Fibroblasts and epithelial cells were separated by differential trypsinization [15]. The purity of epithelial cells was determined by using an anti-multicytokeratin MAb (FK-Biotec, RS, Brazil), and a MAb against human prolyl-4-hydroxylase (Dako, Denmark). Human vaginal epithelial cells (hVECs) were cultured to confluence and subcultured in KM/F12 supplemented with Ham’s F12 nutrient mixture (3:1), fetal bovine serum (10%), insulin (5 μg/ml), transferrin (5 μg/ml), hydrocortisone (0.45 μg/ml), L-glutamine (584 mg/L), Triiodo-L-thyronine (2×10−9M), and penicillin-streptomycin mixture. Only cultures in the first passages were used.
MDCK cells
The MDCK cells ATCC (N° CCL-34) were cultured in 25-cm2 flasks with D-MEM (Dulbecco modified eagle medium) supplemented with 10% fetal calf serum at 37°C, with passages every 48 h.
Parasites
The JT strain of Trichomonas vaginalis was isolated at the Hospital Universitário, Universidade Federal do Rio de Janeiro, Brazil, and has been maintained in culture for several years. This isolate was artificially infected with Mycoplasma hominis as described below. The T016 strain is a fresh isolate kindly provided by Dr. John F. Alderete (Department of Microbiology, University of Texas Health Science Center, USA), presenting high virulence. Tritrichomonas foetus, K strain, a cattle parasite isolated in Rio de Janeiro, Brazil, was used as control.
The cells were cultivated for 24 h at 36.5°C, which corresponds to the end of the logarithmic phase of growth. All strains were cultivated in Trypticase - yeast extract - maltose (TYM) medium [16] supplemented with 10% fetal calf serum, for 24 h. These cultures were then centrifuged at 150g for 5 min, resuspended in TYM medium and used in interaction assays. The investigation for bacterial contamination was performed by DAPI, TEM, antibodies anti-mycoplasmas and PCR using the VenorGen® detection kit (Sigma, USA).
Mycoplasma hominis
M. hominis was purchased from ATCC (N° 23114), and was first cultured in horse serum for 24 h. The culture was centrifuged at 3,000g for 10 min, and the supernatants were filtered through a 0.45 μm-pore-size filter membrane. Aliquots of the filtered supernatant were inoculated in horse serum and then incubated at 37°C in horse serum or D-MEM supplemented with 10% horse calf serum. The bacterial contamination control was performed by PCR using the VenorGen® detection kit. Afterwards, M. hominis was co-incubated in T. vaginalis JT culture in a proportion of 5% (v/v). For other experiments, cultures were treated with antibiotics: 250 μg/ml Amikacin (Bristol-Myers Squibb), and/or 10μg/ml Ciprofloxacin (BioChimico) for 2 h in order to eliminate extracellular bacterial contaminants.
Saccharomyces cerevisiae
Saccharomyces cerevisiae was grown in warm water (37°C) from yeast powder (Fleischmann, Jundiaí, SP, Brazil). The cells were washed three times with warm sterile PBS (phosphate buffer saline), pH 7.2, centrifuged for 5 min at 3,000g at room temperature, and then resuspended in fresh TYM medium to obtain a concentration of 5 × 107 cells/ml. The yeast cells were used immediately.
Determination of in vitro antibiotic susceptibility
The MICs (minimal inhibition concentration) of Gentamicin, Ciprofloxacin and Amikacin for M. hominis isolate was determined as previously described [17].
All samples were incubated at 37°C. Every 24 h, 100 μl of each broth culture was harvested and centrifuged at 20,000g for 20 min. The pellet was then washed once in phosphate-buffered saline (PBS) and resuspended in fresh antibiotic-free medium. Bacterial growth was determined over the next 15 days, by evaluating both the induction of color change of the D-MEM media and DAPI staining in fluorescence microscopy. The mycoplasma-free T. vaginalis JT isolate was cultivated for 1 week in the presence of the same concentrations of antibiotics to assess the absence of any toxic effects of the antibiotics.
Epithelial cells/parasite interaction procedures
The epithelial cells were grown in sterile plastic bottles until confluence. For interaction assays, culture tubes with 106 cells/ml were chilled for parasite detachment and centrifuged at 1,500g. The supernatant was discarded and the pellet resuspended in interaction medium (KM/F12: TYM 2:1). The interaction assays were performed using 10:1 parasites to epithelial cells for different lengths of duration. Cells were treated with Gentamicin to avoid external bacterial contamination.
Fluorescence microscopy
DAPI
In order to check for the presence of mycoplasmas and also for possible morphological changes in the nucleus, cells were stained with 5 μg/ml DAPI (Molecular Probes, USA) for 15 min, and examined in an Axiophot II Zeiss microscope. Images were acquired with a Hamamatsu chilled CCD camera C5985, and processed using Adobe Photoshop (Adobe, USA).
Immunofluorescence
Parasites were fixed with 4% paraformaldehyde in 0.1M sodium phosphate buffer, pH 8.0, and allowed to adhere to poly-L-lysine coated glass coverslips. Both the epithelial cells and trichomonads were treated with 3% Nonidet P-40 for 40 min and then with cold acetone for 15 min. Next, the fixed cells were quenched using 50 mM ammonium chloride, and 3% bovine serum albumin in PBS (BSA/PBS). The cells were incubated overnight at 4°C, with the following monoclonal antibodies: (1) monoclonal anti-human-IV, V, VI, VII, X, XIII, XVIII cytokeratin antibodies (FK-Biotec, RS, Brazil) for hVECs; and (2) monoclonal anti-human prolyl-4-hydroxylase antibody (DAKO-Denmark) specific for fibroblasts. Cells were also stained with 5 μg/ml DAPI for nucleus staining. Cells were washed, incubated with anti-mouse antibody- rhodamine-conjugated, and examined as described in 2.4.1.
Scanning electron microscopy
After parasite interaction with the epithelial cells, samples were fixed in 2.5% glutaraldehyde in 0.1M sodium cacodylate, pH 7.2, and post-fixed with 1% OsO4. Afterwards, the cells were washed in PBS, post-fixed for 5 min in 1% OsO4, dehydrated in ethanol, critical point dried with CO2, and sputter-coated with gold-palladium. The samples were examined in a JEOL 5800 scanning electron microscope.
Transmission electron microscopy (TEM)
After parasite interaction, cells were fixed as described above, post-fixed in 1% OsO4 in cacodylate buffer and 0.8% potassium ferricyanide for 30 min, dehydrated with acetone, and embedded in Epon. Ultra-thin sections were stained with uranyl acetate and lead citrate and observed in a JEOL 1210 electron microscope.
Amoeboid transformation
T. vaginalis amoeboid transformation rate was determined after 60 min of interaction of parasite with hVECs. Trichomonad size and shape were analyzed in 100 random fields using a Zeiss inverted microscope. Results were expressed in percentages. Three independent duplicate experiments were performed.
Trypan blue cell viability assay
The epithelial cells, such as hVECs and MDCK, were subcultured in 24-well plates, and Trypan blue cell viability assay was performed when confluence was reached. At this time, each well contained approximately 2×105 cells/ml. Parasites were added, and viability was analyzed after 1 h, 2 h, 4 h, and 24 h. The following strains/isolates were used: T. vaginalis JT (control, non-infected cells), T. vaginalis JT artificially contaminated with M. hominis, and T. foetus (control). After interaction, the epithelial cells were detached by trypsinization, centrifuged and resuspended in a 0.4% Trypan blue solution in sterile PBS for 1 min. Cell counting was performed in a Neubauer hemocytometer. The epithelial cells incubated only with TYM medium, without trichomonads, were used as control.
Cell viability assay
Epithelial cells were incubated with T. vaginalis (either M. hominis infected or mycoplasma-free), and allowed to interact in a cell ratio of 10:1 parasites:epithelial cell, for different times at 37°C. For control experiments, parasites were not added to the epithelial cells and additional control was performed with the incubation of M. hominis to MDCK culture. At the end of the incubation periods, the remaining cells were fixed with 2% (wt/v) paraformaldehyde, washed in PBS and stained with 0.13% crystal violet 1. The stained material was read in a spectrofluorimeter at a wavelength of 570 nm. Cytotoxicity was calculated as 1 - (E/C); i.e., all measurements of experimental (E) samples (A570) were indexed to those of control (C) samples (E/C), which showed no loss of cells from the well and subtracted from 1.0.
Phagocytosis assays
T. vaginalis (strain JT and JT infected with M. hominis -) were co-incubated with yeast cells, in a ratio of parasite:yeast of 1:20, for 2 h at 37°C as described previously [18]. Three preparations were analyzed by differential interferential contrast microscopy (DIC) in order to evaluate the number of T. vaginalis containing yeast cells. Forty-eight microscopic fields were randomly selected, and 1,000 parasites were counted in each individual preparation.
Statistical analysis
The following experiments were statistically analyzed: (1) rate of amoeboid transformation, (2) cell viability assays, and (3) phagocytic activity. Independent counting was pooled when the coefficient of variance could be assumed identical. Statistical significance was evaluated using two-sample t-test (n = number of independent experiments). A P- value of <0.05 was considered significant.
Results
Establishment of human vaginal epithelial cell culture
In order to investigate the cytopathic effects of T. vaginalis harboring mycoplasmas, a human primary vaginal epithelial cell (hVECs) system was used. After confluence, these cells exhibited morphology and growth characteristics similar to in vivo vaginal epithelial cells, such as stratified layers (Fig. 1a), close cell-cell contact (Figs. 1b and 2a), presence of desmosomes (Fig. 1b) and homogeneous population (Fig. 2a). Interestingly, the stratified layers formed presented an organized parabasal and multiple non-cornified layers (Fig. 1a), which were considered analogous to native human vaginal–ectocervical tissue, remarkably distinguishing it from previous described cell culture models, such as MDCK. These characteristics were maintained during six culture transfers. The hVECs were positive for cytokeratin showing the epithelial nature of vaginal primary cultures and negative for mycoplasmas (not shown).
General views of hVECs. a Scanning electron micrograph of primary hVECs, showing three distinct cell layers (c1, c2, c3), as evidence of culture stratification and cellular differentiation. Note that the upper layer is characterized by the presence of squamous cells (c1). Bar = 2 μm. b TEM of primary hVECs. Note that these cells present regular shape and a close cell-cell contact. Desmosomes are also observed (arrows). Bar = 1.5 μm. c TEM of interaction of hVECs with T. vaginalis no-infected with M. hominis after 1 h of co-incubation. The hVECs exhibit normal sub-structure with healthy mitochondria (M). A tight adhesion between both cells are observed. C Costa; H hydrogenosome, N nucleus. Bar = 1 μm. d TEM of interaction of hVECs with T. vaginalis infected with M. hominis after 1 h of co-incubation. The hVECs exhibit intense vacuolization and several myelin-like figures (arrows). Bars = 700 nm
MIC for M. hominis
In order to assess whether M. hominis is associated with the membrane, is able to reside inside T. vaginalis cells, or was cleared from the infected protozoa, cells were treated with antibiotics as described above. Gentamicin proved to be bactericidal in either DEMEM or horse serum medium at a concentration of 3.125 μg/ ml, while MIC of Ciprofloxacin was 2.5 μg/ ml and 62.5 μg/ ml for Amikacin. After gentamicin treatment, DAPI fluorescence and electron microscopy revealed the absence of mycoplasmas in the extracellular medium.
Interaction assays T. vaginalis × hVECs
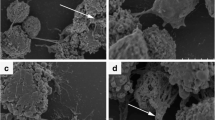
Trichomonas were added in approximately 10:1 ratio of hVECs, over a 45- to 90-min period for electron microscopy analysis. During the interaction, parasites adhered to epithelial cells and aggregated forming clusters (Figs. 2b,c and 3a,b). When mycoplasma-free T. vaginalis were used in interaction experiments the epithelium exhibited less damage (Figs. 2b and 3a) than those T. vaginalis harboring M. hominis (Fig. 2c). Interestingly, parasites harboring mycoplasmas exhibited a more evident amoeboid transformation (Fig. 3b). Moreover, different cell sizes and very distinct shapes could also be observed by mycoplasma-infected trichomonads (Fig. 3c,d).
SEM views of interaction of hVECs with T. vaginalis after 1 h of co-incubation. a T. vaginalis JT, a mycoplasmas-free isolate. b–d T. vaginalis JT artificially infected with M. hominis. The parasites are seen adhered in clusters (a, b), with evident epithelium damage. Note that the parasites are attached even during the mitosis (a, arrows) and their membranes are seen closely apposed (a, c, arrowheads and inset). In c, T. vaginalis infected with M. hominis presented higher rates of ameboid transformation and exhibits an enhanced forms, presenting elongated membrane projections (c, d). Bars: a 10 μm, inset 5 μm; b 20 μm; c, d 10 μm
TEM analyzes extended and confirmed differences in behavior and cytopathic effects provoked by the T. vaginalis used here. Morphologic damage was more intense when parasites were harboring M. hominis (Fig. 1d) when compared with non-infected cells (Fig. 1c). Many hVECs displayed an evident cell death morphology, such as intense cell vacuolization, vacuoles containing myelin-like figures (Fig. 1d), and membrane blebbing.
Epithelial cells viability after T. vaginalis interaction
In order to quantitatively estimate the damage provoked by trichomonas on hVECs, Trypan blue exclusion assay was performed as well the crystal-violet assay. T. vaginalis mycoplasma-free and mycoplasma-infected induced alterations in hVECs viability. However, as seen in Fig. 4, T. vaginalis harboring M. hominis provoked a more intense cytopathic effect. These effects were observed as early as 1 h of interaction, with a striking decrease in hVECs viability within 4 h, and complete epithelial destruction within 24 h when infected trichomonads were used in the interactions assays (Fig. 4). In addition, 100% hVECs destruction in 24 h was only achieved with T. vaginalis harboring M. hominis, whereas T. vaginalis mycoplasma-free left 24% of hVECs viable after 24 h. hVECs without co-incubation with trichomonads remained 97% viable after 24 h. These results were confirmed with crystal violet staining assay (Fig. 5). It is important to point out that mycoplasma-infected T. vaginalis were not able to completely destroy the MDCK monolayer within 24 h, thus causing less damage than to the hVECs.
Comparison of cytotoxicity between T. vaginalis isolates mycoplasmas-free and infected-trichomonas against hVECs. Trypan blue exclusion assay was used to determine cell viability. When hVECs were co-incubated with T. vaginalis harboring mycoplasmas their viability was decreased in the first hours, whereas hVECs with no parasite interaction (control) remained 97% viable after 24 h. The values are expressed as the percentage of living hVECs, and they represent averages of three independent duplicate experiments
Graphic showing the cytotoxicity exerted by T. vaginalis mycoplasmas-infected and mycoplasmas-free when in interaction with MDCK cells. Note that infected T. vaginalis exhibits almost twice damage after 48 h interaction. Controls were performed only with M. hominis in order to exclude possible effects caused by the bacteria
Amoeboid transformation analysis
T. vaginalis harboring M. hominis presented a higher rate of amoeboid transformation when compared to the mycoplasma-free isolates (Fig. 6). In addition, T. vaginalis infected with M. hominis presented amoeboid transformation rates even higher than T. vaginalis T016, which is a fresh isolate. The differences were statistically significant (P < 0.01 by two sample t-test).
Amoeboid transformation of T. vaginalis when co-incubated in the presence of hVECs. T. vaginalis JT is mycoplasma-free (control) is compared with T. vaginalis infected with M. hominis. Note a higher rate of amoeboid transformation in parasites infected with mycoplasmas. The values show the percentage of amoeboid transformation rate by parasites and represent averages of three independent duplicate experiments ± SD. *P-value <0.05 as compared with JT strain of T. vaginalis (two-sample t-test)
Phagocytosis assay analysis
The phagocytic ability (average number of parasites containing yeast cells) was determined in both T. vaginalis mycoplasma-free, and infected with M. hominis (Fig. 7).
Phagocytic analyzes of S. cerevisiae by T. vaginalis. T. vaginalis containing yeast cells in intracellular vacuoles were counted. Analyzes represent averages of three independent triplicate experiments. Note an intense phagocytic activity in trichomonad mycoplasma-infected. ± SD. *P-value <0.05 as compared with JT strain of T. vaginalis
When the strain JT was artificially infected with M. hominis and then used to ingest S. cerevisiae, its phagocytic capacity was found significantly higher (P < 0.05) when compared to the original microorganism-free JT strain.
Discussion
The mechanisms of trichomoniasis infection have been intensely studied [1, 19–21], although the effects of co-infections have been poorly evaluated. It has been proposed that the presence of bacteria could interfere with several cell–cell interactions in both parasites and host cells [22]. In addition, recent reports have proposed a different association of mycoplasmas with T. vaginalis [13], although no correlation with parasite virulence was raised. Despite being similar to the cell cultures previously described [23, 24], the hVECs obtained in this study presented unique characteristics such as homogeneity among cell populations, junctional complexes including desmosomes, and also stratification. Their purity and epithelial origin were certified by immunoassays for cytokeratin, and host-specificity was confirmed based on the parasite’s ability to adhere and destroy the hVECs in few hours.
Previous remarks by other authors have pointed out that it might be uncertain whether the virulence factors and cytopathic effects analyzed so far are species-specific, since T. vaginalis is not naturally occurring in most previous models [3]. Therefore, we used primary hVECs as a model. Protozoa co-infected with mycoplasmas displayed a distinct altered behavior and a higher virulence, as seen by higher ameboid transformation rates and a more intense phagocytic capacity. Furthermore, a decrease of hVECs viability and an increase in hVECs intercellular disruption were also evident when infected protozoa were used. Thus, co-infections with T. vaginalis and mycoplasmas provoke extended cytophatogenic effects.
Krieger et al. [25] stated that T. vaginalis could disrupt epithelial layers and reach deeper cells in tissues. In the present study when hVECs were used, realistic evidence was achieved.
Amoeboid transformation has been correlated to trichomonad virulence [26, 27]. Accordingly, we have observed that isolates that exhibited a higher amoeboid transformation rate were also those that provoked severe damage to hVECs, and thus are considered more virulent. Moreover, different phagocytic abilities have been reported for several parasite isolates and a virulence correlation has been established [18, 28, 29]. The phagocytic ability of T. vaginalis isolates to harbor M. hominis was significantly higher, as compared with mycoplasma-free isolates. These results support the hypothesis that T. vaginalis harboring M. hominis presents higher virulence, as compared with mycoplasma-free trichomonads [30].
It is important to point out that the epithelial cells showed morphological characteristics of cell death when exposed to infected T. vaginalis, such as an intense vacuolization, presence of myelin-like figures, membrane disruption. These effects caused by trichomonads infected with M. hominis are similar to those previously observed in viral infections in epithelial cells [31].
No information regarding the role of Mycoplasmas in T. vaginalis pathogenesis has been previously addressed. These relationships may improve the understanding of the factors involved in T. vaginalis virulence and new clinical aspects of the disease when the parasite is associated with other microorganisms.
References
Krieger JN (1990) Epidemiology and clinical manifestations of urogenital trichomoniasis in men, In: Honigberg BM (ed) Trichomonads parasitic in humans. Springer, New York Berlin Heidelberg, pp 235–245
Pindak FF, William A, Gardner JR, Mora de Pindak M (1986) Growth and cytopathogenicity of Trichomonas vaginalis in tissue cultures. J Clin Microbiol 23:672–678
Petrin D, Delgaty K, Bhatt K, Garber G (1998) Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol 11:300–317
Guenthner PC, Secor WE, Dezzutti CS (2005) Trichomonas vaginalis-induced epithelial monolayer disruption and human immunodeficiency virus type 1 (HIV-1) replication: implications for sexual transmission of HIV-1. Infect Immun 73:4155–4160
Sorvillo L, Smith L, Kerndt P, Ash L (2001) Trichomonas vaginalis, HIV, and African-Americans. Emerg Infect Dis 7:927–932
Viikki M, Pukkala E, Nieminen P, Hakama M (2000) Gynaecologcal infections as risk determinants of subsequent cervical neoplasia. Acta Oncol 39:71–75
Nielsen MH, Nielsen R (1975) Electron microscopy of Trichomonas vaginalis Donné: Interaction with vaginal epithelium in human trichomoniasis. Acta Pathol Microbiol Scand Sect 83:305–320
Scholtyseck E, Teras J, Kasakova I, Sethi KK (1985) Electron microscopy observations on the interaction of Mycoplasma fermentans with Trichomonas vaginalis. Z Parasitenkd 71:432–435
Baseman JB, Lange M, Criscimagna NL, Giron JA, Thomas CA (1995) Interplay between mycoplasmas and host target cells. Microb Pathog 19:105–116
Dallo SF, Baseman JB (2000) Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb Pathog 29:301–309
Taylor-Robinson D, Davies HA, Sarathchandra P, Furr PM (1991) Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int J Exp Pathol 72:705–714
Rappeli P, Carta F, Delogu G, Addis MF, Dessi D, Cappucinelli P, Fiori PL (2001) Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility of M. hominis to human cells. Arch Microbiol 175:70–74
Dessi D, Delogu G, Emonte E, Catania MR, Fiori PL, Rappelli P (2005) Long-term survival and intracellular replication of Mycoplasma hominis in Trichomonas vaginalis cells: potential role of the protozoon in transmitting bacterial infection. Infect Immun 73:1180–1186
Xiao JC, Xie LF, Fang SL, Gao MY, Zhu Y, Song LY, Zhong HM, Lun ZR (2006) Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol Res 100:123–130
Gilbert RO, Elia G, Beach DH, Klaessig S, Sing BN (2000) Cytopathogenic effects of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect Immun 68:4200–4206
Diamond LS (1957). The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol 43:488–490
Vancini RG, Benchimol M (2007) Entry and intracellular location of Mycoplasma hominis in Trichomonas vaginalis. Arch Microbiol DOI 10.1007/s00203-007-0288-8
Pereira-Neves A, Benchimol M (2007) Phagocytosis by Trichomonas vaginalis: new insights. Biol Cell 99:87–101
Silva-Filho FC, DeSouza W (1988) The interaction of Trichomonas vaginalis and Tritrichomonas foetus with epithelial cells in vitro. Cell Struct Funct 13:301–310
González-Robles A, Lazaro-Haller A, Espinosa-Castellano M, Anaya-Velazquez, Martinez-Palomo FA (1995) Trichomonas vaginalis: ultrastructural bases of the cytopathic effect. J Euk Microb 42:641–651
Alderete JF, Garza GE (1985) Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun 50:701–708
Rotten S, Naot Y (1998) Subversion and exploitation of host cells by mycoplasmas. Trends Microbiol 6:436–440
Fichorova RN, Desai PJ, Gibson FC, Genco CA (2001) Distinct proinflammatory host responses to Neisseria gonorrhea infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848
Rajan N, Pruden DL, Kaznari H, Cao Q, Anderson BE, Duncan JL, Schaeffer AJ (2000) Characterization of an immortalized human vaginal epithelial cell line. J Urol 163:616–622
Krieger JN, Ravdin JN, Rein MF (1985) Contact-dependent cytopathogenic mechanisms of Trichomonas vaginalis. Infect Immun 50:768–770
Heath JP (1981) Behavior and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis 57:106–117
Arroyo R, González-Robles A, Martínez-Palomo A, Alderete JF (1993) Signaling of Trichomonas vaginalis for amoeboid transformation and adhesin synthesis follows cytoadherence. Mol Microbiol 7:299–309
Juliano C, Cappuccinelli P, Mattana A (1991) In vitro phagocytic interaction between Trichomonas vaginalis isolates and bacteria. Eur J Clin Microbiol Infect Dis 10:497–502
Rendón-Maldonado JG, Espinosa-Cantellano M, González-Robles A, Martínez-Palomo A (1998) Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol 89:241–250
Cirillo JD, Falkows S, Tompkims LS, Bermudez LE (1997) Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infec Immun 65:3759–3767
Maul GG, Negorev D, Bell P, Ishov AM (2000) Properties and assembly mechanisms of ND10, PML bodies, or PODs. J Struct Biol 129:278–287
Acknowledgments
The authors are grateful to Juliana Leal and Renata Martucci (PABCAM) for technical assistance in the work of hVEC cultures. This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Programa de Núcleos de Excelência (PRONEX), Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) and Associação Universitária Santa Úrsula (AUSU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vancini, R.G., Pereira-Neves, A., Borojevic, R. et al. Trichomonas vaginalis harboring Mycoplasma hominis increases cytopathogenicity in vitro. Eur J Clin Microbiol Infect Dis 27, 259–267 (2008). https://doi.org/10.1007/s10096-007-0422-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0422-1