Abstract
A population-based laboratory surveillance was conducted during a six-year period to define the incidence, demographic risk factors for acquisition, and anti-microbial susceptibilities of Serratia species isolates. A total of 715 incident Serratia species isolates were identified for an annual incidence of 10.8 per 100,000 residents; bacteremic disease occurred in 0.9 per 100,000 residents annually. The incidence increased with advancing age and males were at the highest risk. Ninety-two percent of the isolates were Serratia marcescens, and the majority (65%) of incident Serratia species isolates were of community onset. Ninety-five percent of isolates were susceptible to ciprofloxacin, 98% to gentamicin, 98% to trimethoprim/sulfamethoxazole, and >99% to imipenem. No yearly increase in resistance was observed. Serratia species isolation is most commonly of community onset and older patients and males are at increased risk. Despite reports of increasing resistance among Serratia species, the incidence in our region remains at a low stable rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serratia species, most commonly Serratia marcescens, are recognized as occasional causes of hospital-acquired infections, particularly within critical care areas [1–9]. Numerous hospital outbreaks with Serratia species have been reported, with sources related to the contamination of blood products, parenteral infusions and injections, environmental sources such as contaminated faucets and air conditioners, and transmission by healthcare workers [10–17]. The emergence of multi-drug-resistant strains has further added to the medical importance of Serratia species [18, 19]. Although several observational studies investigating Serratia species infections have been reported [4, 20–24], the studies to date have typically been outbreak investigations or have been reviews conducted in selected tertiary care referral hospitals. As a result, the epidemiology of these infections in a non-selected population is not well defined.
Population-based studies, where all incident cases of a disease occurring in a defined geographic region are studied, minimize selection bias associated with hospital- or clinic-based studies [25, 26]. We, therefore, conducted a population-based laboratory surveillance for all Serratia species isolates occurring in a large Canadian health region to define their incidence, associated demographic risk factors, and anti-microbial susceptibilities.
Materials and methods
Study population
The Calgary Health Region (CHR) provides all publicly funded healthcare services to the 1.2 million residents of the cities of Calgary and Airdrie and numerous adjacent surrounding communities, covering an area of more than 37,000 km2. Acute care is provided principally through one pediatric and three major adult hospitals, which have approximately 2,000 acute care beds and 100,000 discharges yearly. With the exception of a few small rural hospitals, all standard microbiology testing from both community and hospital sites in the CHR is performed by Calgary Laboratory Services. All patients with Serratia species isolates identified at hospital- and community-based collection sites within the CHR identified by Calgary Laboratory Services between 1st January 2000 and 31st December 2005 were included in this study. For the purposes of this study, patients were assumed to be CHR residents and were included in the analysis if they were outpatients with Alberta Personal Healthcare numbers with specimens submitted to CHR-based collection sites or if they were admitted to a CHR acute care facility. Since laboratory information without personal identifiers was studied and individual clinical records were not reviewed, specific institutional ethics review was not sought.
Population-based surveillance
A population-based surveillance for all Serratia species clinical isolates obtained from patients in the CHR during the study was performed at Calgary Laboratory Services. Hospitals, nursing homes, physicians’ offices, and community-based outpatient collection sites were included in the surveillance. Once Serratia species isolates were identified, basic laboratory and demographic information was obtained from the regional laboratory information system (PathNet Classic version 306, Cerner, Kansas City, MO). Community onset isolates were classified as those submitted from community-based collection sites, nursing homes, or those within the first two days of admission to an acute care facility. Hospital onset isolates were those first isolated more than two days after hospital admission. Three or more hospital onset cases with the same Serratia species arising from the same hospital unit within a three-month period was deemed to represent a cluster. In order to minimize the bias associated with repeated culturing of specimens from the same episode of clinical disease, only one culture per patient per species per year was included for the assessment of incident cases.
Clinical laboratory testing
Serratia species were isolated using standard techniques; identification and susceptibilities to anti-microbial agents were determined using Vitek Legacy (Vitek AMS; BioMérieux Vitek Systems Inc., Hazelwood, MO) with custom-made anti-microbial susceptibility testing cards and were interpreted using Clinical and Laboratory Standards Institute (CLSI) criteria. At Calgary Laboratory Services, identification is routinely performed to the species level on all clinically relevant isolates, but, occasionally, individual speciation and anti-microbial susceptibility testing is not performed on some non-invasive isolates. In reporting anti-microbial susceptibilities in this study, intermediate susceptibility results were grouped with and reported as resistant, and, where susceptibility testing was not available, rates were reported using the number tested as the denominator. Because chromosomes of Serratia species encode inducible β-lactamases, Calgary Laboratory Services does not report susceptibilities to penicillins and cephalosporins. Bacteremia was defined by the isolation of Serratia species from at least one set of blood cultures. Polymicrobial isolates were those that had non-Serratia species co-isolated in a single clinical specimen.
Statistical analysis
All analyses were performed using Stata version 9.0 (Stata Corp., College Station, TX). Differences in proportions among categorical data were assessed using Fisher’s exact test. Medians with inter-quartile ranges (IQR) were used to describe asymmetrically distributed continuous variables. Incidence rates were calculated using regional demographic data as the denominator. Category-specific risks were calculated and reported as risk ratios (RR) with exact 95% confidence intervals (CI).
Results
During the six years of surveillance, a total of 1,110 Serratia species were isolated from 1,109 Serratia-species-positive clinical specimens obtained from 651 CHR residents. Seven hundred and fifteen incident episodes of Serratia species isolation occurred for an overall annual population incidence of 10.8 per 100,000. No significant seasonal or yearly variation in incidence was observed. Most of the incident isolates were Serratia marcescens (659; 92%), with the remainder being Serratia liquifaciens (27; 4%), Serratia odorifera (7; 1%), Serratia rubidaea (6; 1%), Serratia fonticola (2), Serratia plymuthica (1), and Serratia not speciated (13; 2%). One third (231/715; 32%) of incident cases were polymicrobial.
The majority (466/715; 65%) of incident Serratia species isolates were of community onset, of which, 56 (12%) of these were sent from emergency departments, 74 (16%) from inpatients within the first two days of admission, 25 (5%) were from nursing homes, and 311 (67%) were from community outpatients. Incident hospital onset isolates (n=249) were identified at a median of 12 (IQR; 6–25) days after admission to an acute care hospital. The proportion of incident Serratia species infections that were hospital onset did not significantly vary according to the study year. The overall hospital onset rate was approximately 0.4 per 1,000 inpatient discharges and isolation varied significantly among the four major CHR acute care institutions, as shown in Fig. 1. The 249 hospital onset cases were isolated from a total of 62 different wards in the four hospitals. Twenty-nine were from intensive care areas, of which, 22 (76%) were from hospital A. A total of ten clusters, all Serratia marcescens, were observed, involving a total of 40 patients on eight different wards. Several medical and surgical wards, an adult intensive care unit, and a rehabilitation ward had clusters involving 3–5 patients per cluster. One single surgical ward in hospital A had a total of 25 cases over the six-year period of the study, with 14 of these occurring in clusters.
The median age of patients with incident Serratia species isolates was 65.9 (IQR; 43.6–77.7) years and 410 (57%) were male. There was a significant relationship observed between age and gender and the incidence of Serratia species isolation, with the rates dramatically increasing beyond 60 years of age, as shown in Fig. 2. Although males were only at a slightly higher risk overall as compared to females (12.4 vs. 9.2 per 100,000; RR 1.35; 95% CI; 1.16–1.57; p=0.0001), among those aged 60 and older, males were at nearly twice the risk (65.9 vs. 36.5 per 100,000; RR 1.8; 1.48–2.21; p<0.0001). While the proportion of males and females with community onset isolation was similar (257/410; 63% vs. 209/305; 69%, p=0.11), the median age was significantly higher for those with hospital as compared to community onset isolation [70.4 (IQR; 53.7–79.2) vs. 62.5 (IQR; 39.7–76.6) years; p=0.002].
Bacteremic disease occurred in 57 patients for an incidence of 0.9 per 100,000/year; the species included Serratia marcescens in 50 (88%), Serratia liquifaciens in 4 (7%), Serratia odorifera in 2 (4%), and one was not speciated. Similar to that observed with the overall isolation of Serratia species, the incidence of bacteremic disease also significantly increased with advancing age and after the age 60 in the male gender, as shown in Fig. 3. While no significant overall relationship existed between age and whether bacteremic cases were of community or hospital onset, all five bacteremic cases occurring in children <10 years of age were of hospital onset in infants less than one year of age.
Of the entire collection of 1,110 Serratia species isolates, the most common specimen type was urine in 452 (41%), respiratory in 292 (26%), wounds/soft tissue in 104 (9%), blood in 101 (9%), eye cultures in 37 (3%), catheter tip in 23 (2%), and miscellaneous fluids in 101 (9%). A total of 420 non-Serratia-species organisms were isolated as part of polymicrobial infections with Serratia species; the most common were Enterococcus species in 69 (16%), Pseudomonas aeruginosa in 60 (14%), Staphylococcus aureus in 46 (11%), yeast in 32 (8%), Escherichia coli in 28 (7%), and Klebsiella species in 27 (6%).
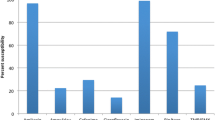
Among incident Serratia species isolates, 638/669 (95%) were susceptible in vitro to ciprofloxacin, 660/673 (98%) to gentamicin, 660/673 (98%) to trimethoprim/sulfamethoxazole, and 425/427 (99.5%) to imipenem. Only 7/668 (1%) were susceptible to nitrofurantoin. There was no overall statistically significant relationship between species and anti-microbial susceptibility observed, although the number of Serratia species other than Serratia marcescens were few. However, among incident isolates, all gentamicin- and trimethoprim/sulfamethoxazole-resistant isolates and 29/31 ciprofloxacin-resistant isolates were Serratia marcescens. The two imipenem-resistant isolates were Serratia marcescens of nosocomial onset, but these isolates remained susceptible to gentamicin, ciprofloxacin, and trimethoprim/sulfamethoxazole. No significant changes in ciprofloxacin, gentamicin, trimethoprim/sulfamethoxazole, or nitrofurantoin resistance occurred during the six years of the study. No association existed between anti-microbial resistance and whether onset was in the community or hospital.
Discussion
This large study represents the first population-based description of the Serratia species and provides novel information on the distribution of bacteremic and non-bacteremic isolates. Serratia species were isolated from approximately 1 in 10,000 CHR residents per year and bacteremia occurred in only 1 in 100,000 annually in this study. Our study confirms that clinical isolates of Serratia species other than Serratia marcescens are rare.
Serratia species are well recognized as occasional causes of nosocomial infections and are frequently associated with outbreaks. They are recognized to be among the most common causes of the contamination of blood products [16], but outbreaks in hospitals have been associated with contaminated water taps [10], air conditioners [11], cleaning solutions [27], improper aseptic technique with medications [12, 14, 15, 17], and inadequate hand and environmental hygiene [2, 6, 13]. We observed several clusters of cases on wards, suggesting nosocomial transmission. However, as part of this study, we neither genotyped the strains to assess relatedness nor collected detailed epidemiologic data to assess clinical relationships between cases. Therefore, our definition of clusters must be recognized as crude, with a significant possibility that we may have either under- or overcalled hospital-based outbreak-related infections.
The recognition that Serratia species are most commonly of community onset is contrary to contemporary understanding and highlights the strengths of population-based designs in defining the epidemiology of an infectious disease. A large number of publications investigating Serratia species have been reports of outbreaks, many which have involved large numbers of patients over prolonged periods of time [2, 4, 11]. Furthermore, multi-centered surveys have indicated that Serratia species are among the top ten causes of nosocomial bloodstream infection [8]. However, unlike previous studies, we included all Serratia species isolated from patients in our region, including from hospitals, physicians’ offices, clinics, nursing homes, and urgent care centers, and attempted to exclude all isolates obtained from non-residents [26]. While we confirm that Serratia species are important among hospitalized patients, through our population-based design, it is evident that these organisms are more commonly isolated from patients in the community. It should be recognized that, however, as a result of our laboratory-based design, we were not able to discern between community-acquired and healthcare-associated community onset disease in this study, and some proportion of our community onset isolates were related to previous hospitalization or medical care [28].
Our observation that the elderly, and, in particular, older males, are at the highest risk (Figs. 2 and 3) for the isolation of Serratia species developing merits discussion. As a proportion of all isolates, patients 60 years and older (280/613; 46%) were more likely to have isolates from urine as compared to younger patients (172/497; 35%). The increased rates of Serratia species isolation in older individuals may, therefore, be in part related to an increased risk for urinary tract infection overall as a result of a higher incidence of incontinence, and urinary tract obstruction and instrumentation associated with advancing age. We did not find any significant differences in the distribution of isolates among elderly males and females, and the excess risk in males remains unexplained. It is possible that males may have been more likely to have samples submitted overall and, therefore, to have positives identified. Indeed, this sampling bias has been recognized to have an influence on incidence rate calculations for cultures from non-sterile sites, although it is much less of a concern for sterile sites, such as for blood and cerebral spinal fluid [29]. Our observation that the risk for Serratia species bacteremia increases with advancing age and that older males are at the highest risk supports the idea that the elderly, and especially older males, are truly at risk for all Serratia species infections.
Increasing resistance to anti-microbial agents has been noted in study series from many countries worldwide, with series documenting the emergence of multi-drug-resistant strains of Serratia species including extended spectrum β-lactamase producers of the CTX-M, TEM, and SHV types [4, 18, 20, 21, 30]. In recent years, the CHR has experienced dramatic increasing rates of resistance to anti-microbials among bacteria, not limited to extended spectrum β-lactamase producing Escherichia coli, metallo-β-lactamase producing Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus [31–33]. It is, therefore, of great interest, and is unexplained, as to why the occurrence of resistance to quinolones, aminoglycosides, and trimethoprim/sulfamethoxazole among Serratia species was low and did not increase in our region over the six-year period of this study. Carbapenem resistance has been reported in Serratia species, usually due to non-metallo carbapenemases or efflux pumps and outer membrane permeability changes in combination with Amp C type enzyme hyperproduction [19, 34]. However, resistance to carbapenems was notably rare in our study, with only two isolates demonstrating reduced susceptibility.
In summary, we present novel data describing the epidemiology of Serratia species isolation in a large non-selected population. We document that, while bacteremic disease is infrequent, the overall isolation of Serratia species is relatively common and the risk increases with advancing age. Although hospital onset outbreaks occur, the majority of Serratia species are of community onset isolation. Resistance to non-β-lactam anti-microbials is low and is not increasing in our region.
References
Kim BN, Choi SI, Ryoo NH (2006) Three-year follow-up of an outbreak of Serratia marcescens bacteriuria in a neurosurgical intensive care unit. J Korean Med Sci 21(6):973–978
Yoon HJ, Choi JY, Park YS, Kim CO, Kim JM, Yong DE, Lee KW, Song YG (2005) Outbreaks of Serratia marcescens bacteriuria in a neurosurgical intensive care unit of a tertiary care teaching hospital: a clinical, epidemiologic, and laboratory perspective. Am J Infect Control 33(10):595–601
Dubouix A, Roques C, Segonds C, Jeannot MJ, Malavaud S, Daude S, Chabanon G, Marty N (2005) Epidemiological investigation of a Serratia liquefaciens outbreak in a neurosurgery department. J Hosp Infect 60(1):8–13
Su L-H, Ou JT, Leu H-S, Chiang P-C, Chiu Y-P, Chia J-H, Kuo A-J, Chiu C-H, Chu C, Wu T-L, Sun C-F, Riley TV, Chang BJ; The Infection Control Group (2003) Extended epidemic of nosocomial urinary tract infections caused by Serratia marcescens. J Clin Microbiol 41(10):4726–4732
Casolari C, Pecorari M, Fabio G, Cattani S, Venturelli C, Piccinini L, Tamassia MG, Gennari W, Sabbatini AM, Leporati G, Marchegiano P, Rumpianesi F, Ferrari F (2005) A simultaneous outbreak of Serratia marcescens and Klebsiella pneumoniae in a neonatal intensive care unit. J Hosp Infect 61(4):312–320
Sarvikivi E, Lyytikäinen O, Salmenlinna S, Vuopio-Varkila J, Luukkainen P, Tarkka E, Saxén H (2004) Clustering of Serratia marcescens infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 25(9):723–729
Ellabib MS, Ordonez A, Ramali A, Walli A, Benayad T, Shebrlo H (2004) Changing pattern of neonatal bacteremia. Microbiology and antibiotic resistance. Saudi Med J 25(12):1951–1956
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39(3):309–317
Bagattini M, Crispino M, Gentile F, Barretta E, Schiavone D, Boccia MC, Triassi M, Zarrilli R (2004) A nosocomial outbreak of Serratia marcescens producing inducible Amp C-type beta-lactamase enzyme and carrying antimicrobial resistance genes within a class 1 integron. J Hosp Infect 56(1):29–36
Horcajada JP, Martinez JA, Alcón A, Marco F, De Lazzari E, de Matos A, Zaragoza M, Sallés M, Zavala E, Mensa J (2006) Acquisition of multidrug-resistant Serratia marcescens by critically ill patients who consumed tap water during receipt of oral medication. Infect Control Hosp Epidemiol 27(7):774–777
Uduman SA, Farrukh AS, Nath KN, Zuhair MY, Ifrah A, Khawla AD, Sunita P (2002) An outbreak of Serratia marcescens infection in a special-care baby unit of a community hospital in United Arab Emirates: the importance of the air conditioner duct as a nosocomial reservoir. J Hosp Infect 52(3):175–180
Ostrowsky BE, Whitener C, Bredenberg HK, Carson LA, Holt S, Hutwagner L, Arduino MJ, Jarvis WR (2002) Serratia marcescens bacteremia traced to an infused narcotic. N Engl J Med 346(20):1529–1537
de Vries JJ, Baas WH, van der Ploeg K, Heesink A, Degener JE, Arends JP (2006) Outbreak of Serratia marcescens colonization and infection traced to a healthcare worker with long-term carriage on the hands. Infect Control Hosp Epidemiol 27(11):1153–1158
Civen R, Vugia DJ, Alexander R, Brunner W, Taylor S, Parris N, Wasserman R, Abbott S, Werner SB, Rosenberg J (2006) Outbreak of Serratia marcescens infections following injection of betamethasone compounded at a community pharmacy. Clin Infect Dis 43(7):831–837
Pan A, Dolcetti L, Barosi C, Catenazzi P, Ceruti T, Ferrari L, Magri S, Roldan EQ, Soavi L, Carnevale G (2006) An outbreak of Serratia marcescens bloodstream infections associated with misuse of drug vials in a surgical ward. Infect Control Hosp Epidemiol 27(1):79–82
Vonberg RP, Gastmeier P (2007) Hospital-acquired infections related to contaminated substances. J Hosp Infect 65(1):15–23
Grohskopf LA, Roth VR, Feikin DR, Arduino MJ, Carson LA, Tokars JI, Holt SC, Jensen BJ, Hoffman RE, Jarvis WR (2001) Serratia liquefaciens bloodstream infections from contamination of epoetin alfa at a hemodialysis center. N Engl J Med 344(20):1491–1497
Cheng KC, Chuang YC, Wu LT, Huang GC, Yu WL (2006) Clinical experiences of the infections caused by extended-spectrum beta-lactamase-producing Serratia marcescens at a medical center in Taiwan. Jpn J Infect Dis 59(3):147–152
Lee HK, Park YJ, Kim JY, Chang E, Cho SG, Chae HS, Kang CS (2005) Prevalence of decreased susceptibility to carbapenems among Serratia marcescens, Enterobacter cloacae, and Citrobacter freundii and investigation of carbapenemases. Diagn Microbiol Infect Dis 52(4):331–336
Shih HI, Lee HC, Lee NY, Chang CM, Wu CJ, Wang LR, Ko NY, Ko WC (2005) Serratia marcescens bacteremia at a medical center in southern Taiwan: high prevalence of cefotaxime resistance. J Microbiol Immunol Infect 38(5):350–357
Naumiuk L, Baraniak A, Gniadkowski M, Krawczyk B, Rybak B, Sadowy E, Samet A, Kur J (2004) Molecular epidemiology of Serratia marcescens in two hospitals in Gdańsk, Poland, over a 5-year period. J Clin Microbiol 42(7):3108–3116
Bouza E, Garcia de la Torre M, Erice A, Cercenado E, Loza E, Rodriguez-Créixems M (1987) Serratia bacteremia. Diagn Microbiol Infect Dis 7(4):237–247
Haddy RI, Mann BL, Nadkarni DD, Cruz RF, Elshoff DJ, Buendia FC, Domers TA, Oberheu AM (1996) Nosocomial infection in the community hospital: severe infection due to Serratia species. J Fam Pract 42(3):273–277
Watanakunakorn C (1989) Serratia bacteremia: a review of 44 episodes. Scand J Infect Dis 21(5):477–483
Schuchat A, Hilger T, Zell E, Farley MM, Reingold A, Harrison L, Lefkowitz L, Danila R, Stefonek K, Barrett N, Morse D, Pinner R; Active Bacterial Core Surveillance Team of the Emerging Infections Program Network (2001) Active bacterial core surveillance of the emerging infections program network. Emerg Infect Dis 7(1):92–99
Laupland KB (2004) Population-based epidemiology of intensive care: critical importance of ascertainment of residency status. Crit Care 8(6):R431–436
Sartor C, Jacomo V, Duvivier C, Tissot-Dupont H, Sambuc R, Drancourt M (2000) Nosocomial Serratia marcescens infections associated with extrinsic contamination of a liquid nonmedicated soap. Infect Control Hosp Epidemiol 21(3):196–199
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Smellie WS, Clark G, McNulty CA (2003) Inequalities of primary care microbiology testing between hospital catchment areas. J Clin Pathol 56(12):933–936
Choi SH, Kim YS, Chung JW, Kim TH, Choo EJ, Kim MN, Kim BN, Kim NJ, Woo JH, Ryu J (2002) Serratia bacteremia in a large university hospital: trends in antibiotic resistance during 10 years and implications for antibiotic use. Infect Control Hosp Epidemiol 23(12):740–747
Laupland KB, Parkins MD, Church DL, Gregson DB, Louie TJ, Conly JM, Elsayed S, Pitout JD (2005) Population-based epidemiological study of infections caused by carbapenem-resistant Pseudomonas aeruginosa in the Calgary Health Region: importance of metallo-beta-lactamase (MBL)-producing strains. J Infect Dis 192(9):1606–1612
Pitout JD, Hanson ND, Church DL, Laupland KB (2004) Population-based laboratory surveillance for Escherichia coli-producing extended-spectrum beta-lactamases: importance of community isolates with blaCTX-M genes. Clin Infect Dis 38(12):1736–1741
Gilbert M, MacDonald J, Gregson D, Siushansian J, Zhang K, Elsayed S, Laupland K, Louie T, Hope K, Mulvey M, Gillespie J, Nielsen D, Wheeler V, Louie M, Honish A, Keays G, Conly J (2006) Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. CMAJ 175(2):149–154
Nakamura T, Shibata N, Doi Y, Okuda K, Nakata C, Heijyo H, Matsuo N, Masuda M, Takahashi H, Arakawa Y (2002) IMP-1 type metalo-beta-lactamase producing Serratia marcescens strains isolated from blood culture between 1991 to 2000. Kansenshogaku Zasshi 76(4):246–253
Acknowledgments
No external financial support was obtained for this study. None of the authors had financial, professional, or personal competing interests that would influence the conduct or reporting of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laupland, K.B., Parkins, M.D., Gregson, D.B. et al. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis 27, 89–95 (2008). https://doi.org/10.1007/s10096-007-0400-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0400-7