Abstract
Despite rather strict recommendations for antibiotic treatment of disseminated Lyme borreliosis (LB), evidence-based studies on the duration of antibiotic treatment are scarce. The aim of this multicenter study was to determine whether initial treatment with intravenous ceftriaxone (CRO) for 3 weeks should be extended with a period of adjunct oral antibiotic therapy. A total of 152 consecutive patients with LB were randomized in a double-blind fashion to receive either amoxicillin (AMOX) 1 g or placebo (PBO) twice daily for 100 days. Both groups received an initial treatment of intravenous CRO 2 g daily for 3 weeks, followed by the randomized drug or PBO. The outcome was evaluated using the visual analogue scale at the follow-up visits. The final analysis included 145 patients, of whom 73 received AMOX and 72 PBO. Diagnoses of LB were categorized as either definite or possible, on the basis of symptoms, signs, and laboratory results. The diagnosis was definite in 52 of the 73 (71.2%) AMOX-treated patients and in 54 of the 72 (75%) PBO patients. Of the patients with definite diagnoses, 62 had neuroborreliosis, 45 arthritis or other musculoskeletal manifestations, and 4 other manifestations of LB. As judged by the visual analogue scale and patient records, the outcome after a 1-year follow-up period was excellent or good in 114 (78.6%) patients, controversial in 14 (9.7%) patients, and poor in 17 (11.7%) patients. In patients with definite LB, the outcome was excellent or good in 49 (92.5%) AMOX-treated patients and 47 (87.0%) PBO patients and poor in 3 (5.7%) AMOX-treated patients and 6 (11.1%) PBO patients (difference nonsignificant, p = 0.49). Twelve months after the end of intravenous antibiotic therapy, the levels of antibodies against Borrelia burgdorferi were markedly decreased in 50% of the patients with definite LB in both groups. The results indicate that oral adjunct antibiotics are not justified in the treatment of patients with disseminated LB who initially receive intravenous CRO for 3 weeks. The clinical outcome cannot be evaluated at the completion of intravenous antibiotic treatment but rather 6–12 months afterwards. In patients with chronic post-treatment symptoms, persistent positive levels of antibodies do not seem to provide any useful information for further care of the patient.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most studies of the efficacy of antibiotics against Borrelia burgdorferi sensu lato focus on the early localized infection, erythema migrans (EM), which is usually easy to treat. Recommendations for antibiotic treatment of disseminated Lyme borreliosis (LB) [1], whether for the selection of antibiotics or the duration and route of administration, are based mainly on clinical experience, along with limited evidence from controlled trials [2, 3]. In clinical practice, various antibiotic treatment regimens have been used in the first-line therapy of disseminated LB [4–6]. In particular, the duration of treatment may vary markedly, and courses of treatment ranging from 2 weeks to several years have been reported [7–9]. Long-standing post-treatment symptoms, or “post-Lyme disease syndrome,” have been treated with antibiotics, but such treatment has had no statistically significant effect beyond that obtained by placebo (PBO) [10, 11]. Delay in the start of antibiotic treatment may increase the frequency of long-standing symptoms. The same primary treatment regimen given years after the initial infection may not be as effective as that given immediately after a timely diagnosis of disseminated LB. Although several antibiotics have shown excellent clinical efficacy [3] in studies of intravenous and oral treatments, treated patients are not always monitored long enough to determine long-term outcomes. Furthermore, no blind studies comparing different durations of antibiotic therapy for disseminated LB have been published in Europe [3].
Ceftriaxone (CRO) is probably the most widely used intravenous antibiotic for treatment of disseminated LB. The aim of this multicenter study was to determine whether an extended period of adjunct oral antibiotic therapy after 21 days of intravenous CRO is beneficial in the treatment of disseminated LB.
Patients and methods
Study population and study design
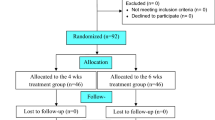
From 1998 to 2003 at the Turku University Central Hospital, the Helsinki University Central Hospital, and the Åland Central Hospital, a total of 152 consecutive patients with disseminated LB were randomized in a double-blind fashion to receive either 1 g amoxicillin (AMOX) or PBO twice daily for 100 days. Both groups were first treated with 2 g intravenous CRO daily for 21 days (Fig. 1). The inclusion criteria were based on a clinical diagnosis confirmed by microbiological tests and the intent-to-treat decisions of the investigators [12]. The exclusion criteria were as follows: allergy to penicillin or cephalosporins, age below 16 years, pregnancy, and receipt of any antibiotic therapy within 1 month of the onset of CRO treatment.
It was calculated that a total of 200 patients (which included an additional 10% of patients to cover possible drop-outs) would have been needed to show a 10% difference in the visual analogue scale (VAS) value (assigned by the patient or the investigator) with an 80% power to detect a significant (p < 0.05, two-sided) difference in the outcome of infection between the groups. The overall standard deviation was estimated to be 2- to 2.5-fold of the difference. Two hundred patients should have been enrolled in the three centers over 2–3 years. However, it took 4 years to enroll 152 patients, and we decided to discontinue enrollment with this number of patients. At the time of patient enrollment, LB was classified as definite or possible (Table 1). This classification was based on symptoms and signs as well as laboratory results. After the start of the treatment and during the follow-up period but before opening of the codes, an initial diagnosis classified as “possible” could be changed to “definite” on the basis of new laboratory results obtained after enrollment.
The following criteria were used for definite Lyme neuroborreliosis (LNB): a classical manifestation of LNB (e.g. facial paresis, meningitis, or meningoradiculitis, along with exclusion of other causes), inflammatory changes in the CSF, and/or intrathecal antibodies against B. burgdorferi, along with exclusion of other causes. The criteria for possible LNB included more uncommon manifestations of LNB, serum antibodies against B. burgdorferi, and exclusion of other causes. The criteria for possible LB with musculoskeletal manifestations were severe arthralgia lasting for at least 3 months and antibodies against B. burgdorferi. For definite LB with musculoskeletal manifestations, objective signs and/or MRI confirmation of arthritis or tendinitis were required in addition to the exclusion of other causes. Detection of B. burgdorferi sensu lato DNA in the synovial fluid also confirmed the diagnosis of definite Lyme arthritis (LA), while the finding of B. burgdorferi DNA in the CSF confirmed LNB.
The duration of symptoms prior to treatment was variable, since the manifestations included both early dissemination and chronic symptoms. The duration of disease is difficult to determine clinically, at least in cases of long-lasting symptoms in which patients cannot exactly detect the duration of slowly progressing symptoms.
Before patients in our study were diagnosed with disseminated LB, an extensive search of any other diagnoses was carried out. The panel of laboratory parameters and tests included sedimentation rate, C-reactive protein level, hemoglobin level, leukocyte and platelet counts, levels of antinuclear antibodies, rheumatoid factor test, liver function tests, and a wide selection of serological tests for other infectious diseases. Serological investigation for syphilis was performed in 84 patients, all with negative results. Rheumatoid factor was positive in 16 of 143 patients. Antinuclear antibodies were elevated before treatment in 8 of the 143 patients but became negative in 4 of them by the end of the follow-up period. Serum ALAT was slightly elevated in 22 of 143 patients at the onset or immediately after the discontinuation of CRO treatment but returned to normal in 13 of them by the end of the follow-up period. The ECG was normal in 125 of 134 patients; of the remaining 9, a first-degree atrioventricular block was detected in 3, a left bundle branch block in 1, signs of a previous myocardial infarction in 1, and frequent ventricular (n = 3) or supraventricular (n = 1) extrasystoles in 4. In patients with neurological symptoms, CSF analysis was frequently used as a diagnostic and differential diagnostic tool: i.e., lumbar puncture was carried out in 124 of 145 patients. Imaging was also used extensively to exclude any other causes, especially in patients with neurological symptoms: an MRI of the brain and/or cervical region was performed in 71 of 145 patients.
Microbiological investigations
The tests for microbiological confirmation of LB included serological investigations, PCR, and culture. Serological testing was initially carried out in each of the three centers. Afterwards, all sera were analyzed at the Department of Medical Microbiology, University of Turku. Serum IgM and IgG antibodies against B. burgdorferi were measured by an in-house enzyme immunoassay (EIA), using fresh antigen technology with sonicated B. burgdorferi sensu stricto organisms as antigen [13–15] and by a commercial EIA using flagellin of B. burgdorferi as antigen (IDEIA; Dako, Glostrup, Denmark). Intrathecal production of antibodies against B. burgdorferi was measured with an LNB kit (Dako). Antibody levels against B. burgdorferi were measured four times after the onset of treatment: immediately, as well as 3, 6, and 12 months after the completion of CRO treatment. A decrease of 20% was considered a moderate decline in antibody concentration, while a decrease of 50% or more was considered a strong decline. Lumbar puncture and measurement of CSF antibody levels and PCR for B. burgdorferi sensu lato was repeated in selected cases during or after treatment. In all 145 patients, an EDTA blood specimen was drawn at the start of CRO treatment and analyzed by PCR for B. burgdorferi sensu lato. In selected patients, CSF and synovial fluid specimens were obtained and analyzed by PCR and culture for B. burgdorferi sensu lato. [15].
Randomization of patients, follow-up of patients, and measurement of outcome
Patients were collected from three centers: Turku University Central Hospital (98 patients), Helsinki University Central Hospital (34 patients), and Åland Central Hospital (13 patients). The randomization was double-blind. The labelling of preparations, including AMOX or PBO, was carried out randomly in the pharmacy of Turku University Central Hospital. The enrolled patients received labelled containers marked with a code. The investigators had no access to the codes before the end of the study.
The study was approved by the ethics committees of the three centers, and the patients signed an informed consent form before enrollment, in accordance with the 1983 revision of the Declaration of Helsinki.
The patients were monitored in the same centers where the initial diagnoses were made. In the largest center, the investigator at each follow-up visit was the same (J.O.), whereas in the other two centers several physicians examined the patients at follow-up visits using a study-specific schedule.
The patients were followed up for 1 year, and their outcomes were measured by the patients themselves and by the investigators using the VAS at the visits at the end of CRO treatment as well as 1, 3, 6, and 12 months later. The VAS value scale ranged from 0 to 100, with the value 50 standing for the baseline before intravenous treatment, 0 for totally symptom free, and 100 for a definitely poor outcome.
Seven patients were withdrawn from the study, five because of discontinuation of the investigated drug and two because of an evident diagnosis other than disseminated LB that was established during the follow-up period.
Statistical analysis
Statistical analyses were carried out using the Mann–Whitney U test to compare patient and investigator VAS values between the groups. The chi-squared or Fisher’s exact test was used to test associations between categorical variables. Logistic regression was used to analyze the decline of the level of antibodies between the AMOX and PBO groups, with generalized logit as a link function. Interpretations were made with odds ratios and are presented with confidence intervals (CIs). A p value lower than 0.05 was considered significant. The statistical analyses were carried out using SAS/STAT(r) software, version 9.1.3 SP4, of the SAS System for Windows. The number of enrolled patients did not reach the target to have sufficient power to make a definite conclusion about the lack of efficacy of the adjunctive treatment.
Results
Characteristics of the patients
The study included 145 patients. The diagnosis of LB was classified as definite in 107 patients and possible in 38. Table 2 shows the symptoms and signs of all patients. Tables 3 and 4 show the symptoms and signs of LNB and LA. Seventy-two patients recalled a tick bite, and 38 patients had had a probable EM. The treatment records of the previous EM allowed classification of 24 cases as adequately treated, while treatment was classified as inadequate in the remaining 14 cases. The codes were opened when the follow-up period for all patients had been completed. After treatment with intravenous CRO, 73 patients (38 women, 35 men) received AMOX and 72 (36 women, 36 men) received PBO. The mean age of patients in the AMOX group was 52.3 years (range, 19–87 years) and 50.5 years (range, 16–80 years) in the PBO group. The diagnosis was definite in 53 (72.6%) AMOX patients and 54 (75.0%) PBO patients and possible in 20 (27.4%) AMOX patients and 18 (25.0%) PBO patients. Of the 107 patients with definite LB, 62 (30 AMOX, 32 PBO) had LNB, 45 (22 AMOX, 23 PBO) had LA, and 4 had other manifestations of the disease. Four patients had both LNB and LA.
Of the four patients with manifestations of definite LB other than LNB or LA, two had acrodermatitis chronica atrophicans (ACA). One patient had myalgia and a secondary EM 17 months after an initial skin lesion that had been treated with oral antibiotics for 2 weeks. PCR assay of a skin biopsy specimen taken from the new lesion (40 cm in diameter) was positive for B. burgdorferi sensu lato. Another patient who had had an EM (measuring 10 × 20 cm) and had been treated with AMOX for 14 days developed systemic symptoms (fever, arthralgia, myalgia, headache, and fatigue) in addition to the skin lesion 45 days before the diagnosis of EM was made. When the systemic symptoms persisted 11 months later, the patient enrolled in the study. These symptoms indicated early dissemination of infection at the EM stage and, presumably, active infection. In addition, serum levels of IgM antibodies against B. burgdorferi were high. The patient became symptom-free 5 days after the start of CRO treatment.
Table 3 shows the main clinical findings and symptoms in LNB patients, while Table 4 shows the main findings in LA patients. The results of CSF analysis were available for 58 of the 62 patients with definite LNB. Intrathecal synthesis of antibodies against B. burgdorferi was detected in 24 patients, lymphocytic pleocytosis in 34 patients, and borrelial DNA by PCR in 2 patients. Brain and/or cervical MRI was done in 71 patients with neurological symptoms; findings were normal in 46 cases, and abnormalities in 20 cases were not directly or clearly attributable to LNB. In the remaining five patients, the findings were compatible with LNB [16]. Follow-up imaging showed improvement in three of four patients examined later. Synovial fluid was analyzed in 17 of the 45 patients with LA. Only one synovial fluid specimen was positive by PCR.
Assessment of clinical outcome
Figures 2 and 3 show the mean VAS values for both patients and investigators, as assessed at the onset of and during treatment as well as during follow-up. Both the investigators and the patients rated the outcomes increasingly better with time after CRO treatment and during follow-up. In both the AMOX and the PBO groups, the mean patient VAS was 30 at the end of CRO treatment and 18 after follow-up (Fig. 2). In both groups, the mean investigator VAS was 34 at the end of CRO treatment and 14 after follow-up (Fig. 3). The mean patient VAS values of AMOX patients with definite LB did not differ significantly from those of PBO patients (p values at the 5 time points: 0.92, 0.20, 0.15, 0.96, and 0.37). Likewise, no significant differences were found between the corresponding mean levels of investigator VAS values (p values at the 5 time points: 0.55, 0.92. 0.58, 0.65, and 0.19), nor were any significant differences found in the two main subgroups, definite LNB and definite LA, between the mean VAS levels of AMOX and PBO patients (data not shown).
Outcome according to patient VAS scores after intravenous CRO treatment at randomization (0) and 1, 3, 6, and 12 months later. VAS scale ranged from 0 to 100, with 50 representing the subjective measure of an outcome similar to that before antibiotic treatment, 0 representing a completely symptom-free outcome, and 100 representing a completely poor outcome
Outcome according to investigator VAS scores after intravenous CRO treatment at randomization (0) to AMOX or PBO treatment and 1, 3, 6, and 12 months later. VAS scale ranged from 0 to 100, with 50 representing the objective measure of outcome similar to that before antibiotic treatment, 0 representing a completely symptom-free outcome, and 100 representing a completely poor outcome
Using VAS values and patient records, the outcomes of all 145 patients assessed for treatment benefit after follow-up were classified as excellent or good (investigator VAS value <30) in 114 (78.6%) patients, controversial (investigator VAS 30–40) in 14 (9.7%), and poor (investigator VAS >40) in 17 (11.7%) (Table 5).
In patients with definite LB, the outcome was excellent or good in 49 (92.5%) AMOX patients and in 47 (87.0%) PBO patients; outcome was poor in 3 (5.7%) AMOX patients and in 6 (11.1%) PBO patients (p = 0.49; Fig. 4). In patients with possible LB, the outcome was excellent or good in 10 (50.0%) AMOX patients and in 8 (44.4%) PBO patients; outcome was poor in 4 (20.0%) AMOX patients and in 4 (22.2%) PBO patients. In the remaining 7 AMOX and 7 PBO patients, the benefits of treatment(s) were doubtful.
In the AMOX group, patients with definite LB showed statistically significantly better outcomes at the last follow-up visit than patients with possible LB (p = 0.03 for patient VAS values, and p = 0.007 for investigator VAS values). For patient VAS values, this difference was already statistically on the borderline of significance (p = 0.05) at 6 months of follow-up. In the PBO group, no statistically significant differences were seen between outcomes of patients with definite versus possible LB measured during the follow-up period.
Microbiological investigations
Three of the 145 study patients were seronegative. One of them was a 37-year-old woman with neurological manifestations, slight meningeal irritation, and high proliferation of peripheral blood mononuclear leukocytes following stimulation with B. burgdorferi. The second patient was a 39-year-old woman with relapsing knee arthritis who was persistently seronegative but whose synovial fluid had shown B. burgdorferi sensu lato DNA by PCR 3 years earlier (she was not treated with antibiotics at that time because the synovitis had been resolved for a prolonged period of time). The third patient, a 63-year-old woman, had had an EM and radiculitis 1 year before enrollment and had been treated insufficiently at that time with just a 10-day course of AMOX. New neurological symptoms developed and an MRI showed nonspecific white-matter lesions. Despite seronegativity after initial seropositivity, she was given intravenous treatment and was enrolled in the study.
None of patients with definite LB were seronegative. The blood specimen of one (0.7%) patient was positive by PCR for B. burgdorferi sensu lato. Different clinical specimens (mainly CSF) of 120 patients were analyzed by PCR. Five (4.2%) of them were positive (2 CSF, 1 synovial fluid, 2 skin biopsy specimens). When cultured, all CSF and synovial fluid samples were negative. Borrelia burgdorferi sensu lato grew from two skin biopsy specimens. One of the patients from whom these biopsies were obtained had a secondary EM, and the other had meningoradiculitis and a small skin lesion on the back with histological features of ACA. Table 6 shows details of the eight patients with culture- or PCR-positive specimens.
The levels of antibodies against B. burgdorferi usually remained stable over the first 3 months after completion of CRO treatment. Of the 73 AMOX-treated patients, a strong decline in antibody levels over a 1-year period was detected in 34 (46.6%), whereas a moderate decline and no decline were detected in 11 (15.1%) and 28 (38.4%), respectively. Of the 72 patients in the PBO group, antibody levels declined strongly in 32 (44.4%), moderately in 7 (9.7%), and not at all in 33 (45.8%). When comparing the patients with a strong decline in antibody levels (66 patients) to those with no decline (61 patients), there was no statistical difference between the AMOX and PBO groups (Fisher’s exact test). The magnitude of these nonsignificant effects was calculated using logistic regression: the odds ratio for strong decline (vs. no decline) of antibody levels in the AMOX versus the PBO group was 1.25 (p = 0.53, CI 0.62–2.52) and for moderate decline (vs. no decline) 1.85 (p = 0.26, CI 0.63–5.42).
Among patients with a definite diagnosis of LB, antibody levels over 12 months decreased strongly (decline of at least 50% of the pretreatment sample value) in 26 (49.1%) AMOX patients and in 27 (50.0%) PBO patients. No decline was seen in 19 (35.9%) AMOX patients or in 23 (42.6%) PBO patients (Fig. 5). Antibody levels decreased strongly in 52 of 96 (54.2%) patients with definite LB and an excellent or good outcome, whereas no decline was seen in 32 (33.3%) of these patients (Table 7). On the other hand, in the nine patients with definite LB and a poor outcome, antibody levels decreased strongly in only one patient, while no decline occurred in eight patients (Table 7). The difference in the decline of antibodies among patients with definite LB between patients with an excellent or a good outcome and patients a poor outcome was statistically significant (p = 0.016). Of the 42 patients with definite LB but no decline in antibodies over a 1-year period, 19 belonged to the AMOX group and 23 to the PBO group (p = NS). Among patients with definite LNB, a strong decline in antibody levels over 1 year was more common (56.7% in the AMOX group and 56.3% in the PBO group; p = NS) than no decline (30.0% in the AMOX group and 37.5% in the PBO group; p = NS), whereas among patients with definite LA, no decline was more common (45.5% in the AMOX group and 52.2% in the PBO group; p = NS) than a strong decline (36.4% in the AMOX group and 39.1% in the PBO group, p = NS). Of the 38 patients with possible LB, antibody levels decreased strongly in 34.2% and remained unchanged in 50%.
Safety
No serious adverse effects of antibiotic treatment(s) occurred in any of the 145 patients. Diarrhea was reported in 33 (22.8%) patients during intravenous CRO treatment and in 19 (13.1%) patients during oral treatment (15 in the AMOX group and 4 in the PBO group, p = 0.012). The diarrhea was usually mild and resolved spontaneously over about 2 weeks. No patient had to discontinue treatment due to diarrhea. One patient in the PBO group who had diarrhea during CRO treatment was treated with metronidazole for Clostridium difficile colitis. Another case of C. difficile colitis was identified in one of the five patients who were withdrawn from study because of discontinued use of the study drug. Cholecystitis or biliary sludging was not observed in any patient, nor did any patient develop urticaria or other allergic reactions to CRO or AMOX.
Drug fever was suspected in two patients; both had a high fever peak after each intravenous dose of CRO during the third week of treatment. However, fever was also reported in several other patients during antibiotic treatment(s). Fever peaks (total number of periods: 17) during treatments occurred in 15 (10.3%) patients; of these peaks, four occurred during PBO treatment, four during AMOX treatment, and nine during CRO treatment. The fever that occurred in one patient during PBO treatment was probably due to pneumonia and was treated with cefadroxil for 10 days. Two patients (1 in the PBO group and another in the AMOX group) probably had a viral illness, with fever lasting 1 and 2 days, respectively. Two patients had unexplained fever during PBO treatment (one for 2 days and another for 1 day). The remaining 10 patients had fever peaks that lasted 1–7 days (mean 3.5 days) during antibiotic treatment. These peaks occurred during CRO treatment in nine patients and during AMOX treatment in three patients. Only two patients had fever on the first day of CRO treatment. In addition to fever, concomitant intensive headache affected two of these patients, and severe pain in the knee occurred in one patient. One patient developed facial paresis 3 days after the onset of CRO treatment. About half of the patients reported transient intensification of symptoms during CRO treatment.
We are aware of six deaths among the study patients, all of them occurring after follow-up at ages ranging from 56 to 90 years. Three deaths occurred 2 years after the treatment (2 of causes unknown to the investigators, 1 of breast cancer), one occurred 4 years (cancer of the pancreas) post treatment, one 5 years (acute myocardial infarction) post treatment, and one 6 years (unknown) post treatment.
Discussion
The VAS assessments of treatment outcome and the analysis of patient records produced no evidence of the usefulness of oral adjunct antibiotic treatment after 21 days of intravenous CRO treatment for disseminated LB, since no significant difference was found between the outcome of patients who received PBO after CRO treatment and those who received AMOX after CRO treatment. The clinical outcome after treatment of disseminated LB cannot be judged at the completion of antibiotic treatment but must instead be assessed 6–12 months after completion of treatment. Although the mean VAS values did not decrease significantly more after 12 months as compared to the values recorded at 6 months, there were, in both groups, individual patients who exhibited a marked decrease in the value only after 1 year of follow-up.
On the other hand, earlier evidence of the efficacy of CRO treatment is so favorable that PBO control of the initial treatment would have been unethical. Our patients had a wide spectrum of manifestations of LB, including chronic LNB, which is considered the most difficult manifestation to treat. Therefore, a 3-week course of CRO treatment was chosen instead of shorter courses, which might have been the optimal choice for some of the other manifestations. For the oral adjunct antibiotic treatment, we chose AMOX instead of, for example, doxycycline, which may have marked photosensitivity as an adverse effect. Furthermore, the penetration of AMOX into perivascular space is probably sufficient for treatment of LNB patients. Our results further support the efficacy of intravenous CRO, since outcomes were excellent or good in 114 of all 145 (78.6%) patients treated with it, whereas only 17 (11.7%) patients received no benefit from the treatment(s). The benefit of treatment(s) in cases of definite disseminated LB was even better.
Dattwyler et al. [17] recently studied the duration of antibiotic treatment of disseminated LB. They compared a 2-week course of intravenous CRO with a 4-week course in a PBO-controlled, open-label study. They concluded that the shorter treatment period was equally efficacious. However, the treatment failed in 5 of 80 patients in the 2-week group, whereas no treatment failures occurred in the 4-week group. The authors wondered whether there was a subgroup of patients who had benefited from the longer treatment. Since the study was not double-blinded and clinical responses were rated in only three categories (cure, improvement, or failure) without VAS assessments, it may have shown treatment failures less efficiently than our study. North American studies of the efficacy of antibiotics and the duration of treatment in disseminated LB are not necessarily comparable with European studies. The prevalence of B. burgdorferi sensu lato genospecies differs markedly between the continents [18], and since the organotropisms of genospecies differ, the manifestations of the disease may differ as well [19, 20]. In Dattwyler’s [17] study, about 90% of the patients had musculoskeletal manifestations and about one-third had neurological symptoms, whereas our corresponding figures were 44% and 79%. However, the overall clinical cure rates were similar (70–80%).
Of our 145 patients, 33 suffered from fatigue (16 in the AMOX group and 17 in the PBO group), but in only one of these patients was it the only symptom. We did not record fatigue as a single symptom after treatment but as part of the total VAS-assisted evaluation of outcome. The high prevalence of excellent or good outcomes after intravenous CRO treatment shows that the fatigue experienced usually disappeared after therapy. Krupp et al. [21] studied the treatment of patients with post-treatment Lyme disease syndrome. They concluded that fatigue was relieved by CRO treatment, although there was no difference between the CRO group and the PBO group in cognitive functions or laboratory values. They did not recommend 4 weeks of CRO therapy for patients with post-treatment Lyme disease syndrome because it was associated with a high frequency of adverse effects. Klempner et al. [10] and Kaplan et al. [22] carried out two double-blind, PBO-controlled studies of patients with either seropositive or seronegative post-treatment Lyme disease syndrome. Antibiotics had no beneficial effect, and seropositive and seronegative patients had similar outcomes. The above three studies only included patients who had been treated earlier with antibiotics and were retreated months to several years later due to persistence of symptoms. Therefore, these studies are not readily comparable to our study, since all of our patients received similar treatment regardless of whether they had symptoms after intravenous CRO treatment.
Four patients had both LNB and LA. They did not differ from other patients in outcome or decline of antibody levels. Three of them had an excellent response to treatment. One of them was seronegative in all samples tested, but his CSF was positive by PCR. One (PBO group) exhibited a strong decline in antibody levels, one (AMOX group) exhibited no decline in antibody levels, and one (PBO group) had a poor clinical outcome and no decline in antibody levels.
Live microbes or their DNA or antigens can be regarded as conclusive signs of an active infection. When clinical specimens other than skin are investigated, culture of B. burgdorferi sensu lato or detection of B. burgdorferi DNA by PCR is very rarely positive [14, 20]. Despite extensive investigations, the number of patients in whom B. burgdorferi sensu lato or its DNA could be detected remained very low in our study (Table 5). Since we only enrolled cases of disseminated LB and since secondary EM is very rare in Europe, only a few skin biopsy specimens were analyzed.
The finding that AMOX-treated patients with definite LB had significantly better outcomes than those with possible LB indicates that our diagnostic classification was relevant. In patients with definite disseminated LB, the efficacy of intravenous CRO therapy was evident but did not probably depend on the adjunct oral AMOX treatment.
Diarrhea was the most common adverse event, but it usually disappeared over 1–3 weeks without discontinuation of the antibiotic and without additional medication. Clostridium difficile infections were rare, and no severe allergic reactions were observed. Mild adverse effects (e.g. rash and fever) and intensification of LB symptoms (e.g. arthralgia, headache) during antibiotic treatment may be caused by reactive components released from dying spirochetes. It has been estimated that Jarisch–Herxheimer-like reactions occur in 15% of LB patients within the first 24 h of antibiotic treatment. However, in our study, adverse effects and worsening of symptoms occurred in a considerably greater proportion of patients and also occurred later (second and third week of CRO treatment) than reported previously. In particular, the prevalence of fever was unexpectedly high, since 10% of all patients had fever peaks, often recurring for several days. We suggest that Jarisch–Herxheimer-like reactions may be prolonged and may occur late during treatment.
At 6–12 months after the end of intravenous antibiotic therapy, the level of antibodies against B. burgdorferi decreased markedly in 50% of the patients with definite LB in both the AMOX group and the PBO group. Patients with a poor clinical outcome had persistently high antibody levels significantly more frequently than those with a good outcome. The lack of decline in antibody levels was more common in the PBO group than in the AMOX group. However, this difference in antibody levels between the treatment arms did not reach statistical significance. It can be concluded that decreased antibody levels are an indicator of successful therapy. However, it cannot be concluded that a patient with a poor clinical outcome and persistently high antibodies has a chronic infection. The vast majority of patients with persistently high antibodies had an excellent or good clinical outcome. Our serological findings are consistent with the findings of several previous studies and support published recommendations about the use of serology in treated patients [4]. In patients with chronic post-treatment symptoms, persistent positive levels of antibodies do not seem to provide any useful information for further care of the patient.
In conclusion, our results indicate that oral adjunct antibiotics are not beneficial in the treatment of patients with disseminated LB who initially receive a 3-week course of intravenous CRO.
References
Wormser GP, Dattwyler RJ, Shapiro ED et al (2006) The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134
Steere AC, Schoen RT, Taylor E (1987) The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731
Dinser R, Jendro MC, Schnar S, Zedler H (2005) Antibiotic treatment of Lyme borreliosis: what is the evidence? Ann Rheum Dis 64:519–523
Steere AC (2001) Lyme disease. N Engl J Med 345:115–125
Logigian EL, Kaplan RF, Steere AC (1990) Chronic neurologic manifestations of Lyme disease. N Engl J Med 323:1438–1444
Kristoferitsch W, Baumhackl U, Sluga E, Stanek G, Zeiler K (1987) High-dose penicillin therapy in meningopolyneuritis Garin–Bujadoux–Bannwarth. Clinical and cerebrospinal fluid data. Zentralbl Bakteriol Mikrobiol Hyg [A] 263:357–364
Ziska MH, Donta ST, Demarest FC (1996) Physician preferences in the diagnosis and treatment of Lyme disease in the United States. Infection 24:182–186
Wahlberg P, Granlund H, Nyman D, Panelius J, Seppälä I (1994) Treatment of late Lyme borreliosis. J Infect 29:255–261
Donta ST (1997) Tetracycline therapy for chronic Lyme disease. Clin Infect Dis 25(Suppl 1):52–56
Klempner MS, Hu LT, Evans J et al (2001) Two controlled trials of antibiotic treatment in patients with persistent symptoms, and a history of Lyme disease. N Engl J Med 345:85–92
Sigal LH (2002) Misconceptions about Lyme disease: confusions hiding behind ill-chosen terminology. Ann Intern Med 136:413–419
Stanek G, O’Connell S, Cimmino M et al (1996) European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr 108:741–747
Viljanen MK, Punnonen J (1989) The effect of storage of antigen-coated polystyrene microwells on the detection of antibodies against Borrelia burgdorferi by enzyme immunoassay (EIA). J Immunol Methods 124:137–141
Oksi J, Uksila J, Marjamäki M, Nikoskelainen J, Viljanen MK (1995) Antibodies against whole sonicated Borrelia burgdorferi spirochetes, 41 kilodalton flagellin and P39 protein in patients with PCR- or culture-proven late Lyme borreliosis. J Clin Microbiol 33:2260–2264
Oksi J, Marttila H, Soini H, Aho H, Uksila J, Viljanen MK (2001) Early dissemination of Borrelia burgdorferi without generalized symptoms in patients with erythema migrans. APMIS 109:581–588
Halperin JJ, Luft BJ, Anand AK, Rogue CT, Alvarez O, Volkman DJ, Dattwyler RJ (1989) Lyme neuroborreliosis: central nervous system manifestations. Neurology 39:753–759
Dattwyler RJ, Wormser GP, Rush TJ et al (2005) A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien Klin Wochenschr 117:393–397
Baranton G, Postic D, Saint-Girons I et al (1992) Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS 461 associated with Lyme borreliosis. Int J Syst Bacteriol 42:378–383
van Dam AP, Kuiper H, Vos K et al (1993) Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis 17:708–717
Strle F, Ruzic-Sabljic E, Cimperman J, Lotric-Furlan S, Maraspin V (2006) Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis 43:704–710
Krupp LB, Hyman LG, Grimson R et al (2003) Study and treatment of post Lyme disease (STOP-LD). A randomised double masked clinical trial. Neurology 60:1923–1930
Kaplan RF, Trevino RP, Johnson GM et al (2003) Cognitive function in post-treatment Lyme disease. Do additional antibiotics help? Neurology 60:1916–1922
Acknowledgements
The authors thank Bristol–Myers Squibb for their AMOX tablets and Roche for covering part of the costs of the study. The language of the manuscript was revised by Simo Merne, MA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oksi, J., Nikoskelainen, J., Hiekkanen, H. et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: a double-blind, randomized, placebo-controlled, multicenter clinical study. Eur J Clin Microbiol Infect Dis 26, 571–581 (2007). https://doi.org/10.1007/s10096-007-0340-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0340-2