Abstract
The study presented here determined the relationship between antimicrobial resistance in Streptococcus pneumoniae and the use of antimicrobial agents in 15 different European countries. Pneumococcal isolates (n = 1974) recovered from patients with community-acquired respiratory tract infections during the winter of 2004–2005 in 15 European countries were characterized. The overall percentages of isolates demonstrating intermediate or complete resistance to penicillin, erythromycin, tetracycline, trimethoprim-sulfamethoxazole (TMP-SMX) and ciprofloxacin were 24, 24.6, 19.8, 26.7 and 2%, respectively, as determined using the broth microdilution MIC method recommended by the Clinical and Laboratory Standards Institute. The overall and mean antimicrobial consumption levels (ACL)—i.e., the defined daily doses per 1,000 inhabitants per day—were obtained from the European Surveillance of Antimicrobial Consumption project for each of the 15 countries for the years 1998–2004. Using linear regression analysis, the mean annual ACL for β-lactams, macrolides, tetracyclines, TMP-SMX and fluoroquinolones in each country was compared to the country-specific resistance rates determined in 2004–2005. The rate of overall antimicrobial use in all 15 European countries was significantly associated with antimicrobial resistance in S. pneumoniae. There was variation among the different antimicrobial classes as drivers of resistance, with β-lactams having the strongest association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is the most important bacterial cause of community-acquired respiratory tract infection (CARTI) [1, 2]. Antimicrobial resistance in S. pneumoniae began to emerge in some parts of the world during the late 1970s [3]. Since then, it has developed into a major public-health problem worldwide [1, 4–6], and the effective management of CARTIs has become increasingly difficult. In 2005, results reported from a multinational surveillance study, the Global Respiratory Antimicrobial Surveillance Project (GRASP), confirmed the widespread problem of antimicrobial resistance in S. pneumoniae in multiple European countries [4]. Several previous studies showed geographic differences in the patterns of antimicrobial resistance in Europe, with rates generally being lower in the northern European countries compared to those in southern Europe [4, 7, 8]. Furthermore, antimicrobial use has been identified increasingly as the main selective pressure driving the emergence of resistance [7, 9].
The European Surveillance of Antimicrobial Consumption (ESAC) project is an international surveillance network that provides comparable and reliable data on antimicrobial use in Europe. The highest rates of antimicrobial consumption have been found to occur in the primary-care setting among patients with CARTIs [7]. The importance of assessing antimicrobial resistance in the context of usage has been increasingly recognized and surveillance systems like the ESAC network continue to enhance their databases to facilitate correlation studies. While earlier studies investigating the relationship between antimicrobial usage and resistance have commonly been conducted in settings confined to a single hospital, patient group or country [9, 10], it is important to monitor antimicrobial resistance and usage data on national levels and in comparison with other countries. The purpose of the present study was to compare the rates of antimicrobial resistance in S. pneumoniae to the antimicrobial usage profiles in 15 European countries.
Materials and methods
Unique isolates of S. pneumoniae (n = 1974) were obtained from patients with a variety of different CARTIs in 47 participating centers in 15 European countries between September 2004 and May 2005. The numbers of participating test centers and the numbers of isolates submitted from each country were as follows: France (7/309), Germany (4/185), Italy (5/208), Spain (5/232), Greece (1/7), The Netherlands (3/124), Sweden (4/170), Norway (2/72), Finland (2/91), Denmark (2/89), UK (3/135), Poland (4/137), Slovenia (2/103), Slovak Republic (1/44), and Croatia (2/68). The isolates were shipped to a central referral laboratory (Instituto Valenciano de Microbiología, Valencia, Spain) where isolate identification was confirmed. Stock cultures were then prepared, frozen at −70°C, and shipped on dry ice to the central testing laboratory at the University of Iowa, Iowa City, IA, USA. There, the identification was again confirmed and the isolates were stored at −70°C until further testing.
Susceptibility testing was performed using broth microdilution according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [11]. The following five antimicrobial agents were tested: penicillin, erythromycin, tetracycline, trimethoprim-sulfamethoxazole (TMP-SMX) and ciprofloxacin. Published CLSI breakpoints were used to define susceptibility where available [11]. No CLSI breakpoints for ciprofloxacin against S. pneumoniae were available. Therefore, reduced susceptibility to ciprofloxacin was defined as a ciprofloxacin MIC of ≥4 mg/dl [12]. Multidrug resistance (MDR) was defined as penicillin resistance (including intermediate) plus resistance to at least two other antimicrobial classes.
Data regarding antimicrobial use in Europe, compiled by ESAC, were made available for the purpose of this study by two of the authors who are affiliated with the organization (H.G. and M.F.). Data regarding systemic antimicrobial agents used for ambulatory care were collected in accordance with the anatomic therapeutic chemical (ATC) classification and defined daily dose (DDD) measurement unit (WHO, version 2005) [13, 14]. Adherence to the ATC/DDD 2005 guidelines (upgraded version) was mandatory because of ongoing updates to the database to include new data. A complete description of the organizations providing the data and details of the methodology and validity of the data collected were published previously by other researchers [15]. Usage data for the period 1998–2004 were collected as DDD/1000 inhabitants/day (DID) for each country and antimicrobial class. The average (mean) antimicrobial consumption level (ACL) for each antimicrobial class in each participating country was determined for the entire time period (1998–2004). Comparison of antimicrobial usage data (mean ACL values) and antimicrobial resistance data (2004–2005) was performed by linear regression analysis using SAS software v9.1.3 (SAS Institute, Cary, NC, USA).
Results and discussion
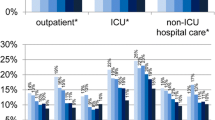
The mean levels of antimicrobial consumption for each country are illustrated in Fig. 1. No significant year-to-year variations in antimicrobial usage levels were noted for the individual countries during the period 1998–2004. Therefore, further data analysis was performed using the average annual ACL values for the entire 6-year period. Striking geographic differences were noted for total outpatient antimicrobial use in these 15 European countries, varying by a factor of 3.2 between the country with the highest DID (France, 31.6) and the country with the lowest DID (The Netherlands, 9.7). Beta-lactam antimicrobials, primarily penicillins, were the agents prescribed most commonly in all countries participating in this study, ranging from 40% (The Netherlands) to 67% (Croatia and the Slovak Republic). However, significant variations were noted among countries in the prescription of fluoroquinolones, macrolides and TMP-SMX. Total macrolide use varied by a factor of 8.0 between the country with the highest use (Greece, 7.5 DID) and the country with the lowest use (Sweden, 0.9 DID). Similarly striking was the difference in fluoroquinolone use, which varied by a factor of 13.7 between the country with the highest use (Italy, 2.8 DID) and the country with the lowest use (Denmark, 0.2 DID). As described previously [7], significant differences in antimicrobial consumption in several European countries are noted consistently, with ACL generally being lower in northern European countries and higher in southern European countries. An increased use of broad-spectrum antimicrobial agents was found in southern European countries.
Rates of antimicrobial resistance in S. pneumoniae are shown in Table 1. A total of 1,974 S. pneumoniae isolates were examined. Overall, 24% of all isolates had decreased susceptibility (intermediate and resistant) to penicillin, 24.6% had decreased susceptibility to macrolides, and 15.8% showed multidrug resistance. Rates of penicillin resistance ranged from no observed resistance in Denmark to 57.1% resistance in Greece. Rates of macrolide resistance ranged from 6.9% in Norway to 57.1% in Greece, and multidrug resistance ranged from none observed in Denmark to 42.9% in Greece. Similar differences were observed for the other antimicrobial agents tested. Overall, resistance rates were much lower in northern European countries (Norway, Sweden, Denmark and The Netherlands) and higher in southern and eastern European countries (Greece, Italy, France and the Slovak Republic). Determined in 2004–2005, these resistance patterns were similar to those noted in Europe in the GRASP study for the years 2002–2003 [4]. Modest increases in the rates of pneumococcal resistance against penicillin, macrolides, fluoroquinolones and MDR were observed in Greece, Italy, the Slovak Republic and Slovenia; however, the changes between 2002–2003 and 2004–2005 were not statistically significant. No single country was consistently in the lowest or highest quartile of resistance for every antimicrobial agent tested. However, The Netherlands, Denmark, Norway and the UK were most often in the lowest quartile, while the Slovak Republic, Italy, Greece and France were most often in the highest quartile of resistance.
Results of linear regression analysis comparing levels of antimicrobial usage in the years 1998–2004 and the rates of antimicrobial resistance in S. pneumoniae in 2004–2005 are presented in Table 2. A significant association between antimicrobial usage and S. pneumoniae resistance was identified for β-lactam agents and resistance to penicillin (R 2 = 0.79), erythromycin (R 2 = 0.66), tetracycline (R 2 = 0.74), TMP-SMX (R 2 = 0.67) and MDR (R 2 = 0.78). Macrolide use was significantly associated with MDR (R 2 = 0.63) and erythromycin (R 2 = 0.79) resistance. Total antimicrobial use was significantly associated with resistance to penicillin (R 2 = 0.79), erythromycin (R 2 = 0.78), tetracycline (R 2 = 0.80), TMP-SMX (R 2 = 0.65) and MDR (R 2 = 0.84). Figure 2a and b graphically depict the relationship between β-lactam use and penicillin resistance and between macrolide use versus macrolide resistance in S. pneumoniae in the 15 participating countries.
Levels of tetracycline and TMP-SMX use were not significantly associated with resistance to any antimicrobial class, including resistance to tetracycline in the case of tetracycline usage or TMP-SMX resistance in the case of TMP-SMX usage. The latter observation may be explained by the consistently low levels of TMP-SMX use throughout Europe during the period surveyed (Fig. 1). An explanation for the lack of association between tetracycline use and resistance remains elusive. In general, the levels of tetracycline use were roughly comparable to those of macrolide use; indeed, in some countries, substantially more tetracycline was used (Fig. 1). The reason why macrolide use would be associated with resistance while tetracycline use is not is presently unknown.
In no instance was the use of any antimicrobial class significantly associated with fluoroquinolone resistance in S. pneumoniae. This may be explained by the generally low levels of fluoroquinolone use in Europe during the period studied together with the relatively low levels of fluoroquinolone resistance observed. Given the study design, it would be difficult to demonstrate a relationship between use and resistance until both the levels of use and the rates of resistance increased to a high enough level. That said, the one country with the highest rate of fluoroquinolone resistance (Italy, 7.2%; Table 1), also had the highest level of fluoroquinolone use (Fig. 1).
Countries with higher levels of overall antimicrobial use demonstrated an increased prevalence of antimicrobial resistance. France had an overall DID of 31.6 and resistance rates of 49.2% for penicillin, 50.1% for erythromycin and 40.8% for MDR. Greece had an overall DID of 29.5 and resistance rates of 57.1% for penicillin and erythromycin and 42.9% for MDR. In contrast, countries with lower levels of overall antimicrobial use generally had lower rates of resistance. For example, the Netherlands had a DID of 9.7 and resistance rates of 4% for penicillin, 11.3% for erythromycin and 3.2% for MDR.
In conclusion, this study found a significant association between the levels of antimicrobial use and the rates of antimicrobial resistance in S. pneumoniae in 15 European countries. Moreover, variations in antimicrobial use in some of the 15 European countries surveyed were more pronounced than in other countries. Clearly, antimicrobial prescribing practices vary among these countries. Higher rates of resistance in countries that use more antimicrobial agents overall underscore the role antimicrobial use plays as a driver of resistance. Moreover, different antimicrobial agents appear to exert different levels of selective pressure. Beta-lactam use appeared to be associated most strongly with resistance to several individual antimicrobial classes and to MDR.
Our findings support previously published data that suggested a correlation exists between antimicrobial use and resistance [7, 9]. As interventions are developed to manage the problem of antimicrobial resistance in S. pneumoniae, data relating usage profiles to resistance will become increasingly important. Such data will provide valuable information upon which changes in prescribing practices can be based. Carefully considered, comprehensive, systematic strategies should be developed and implemented with the aim of reducing overall antimicrobial use in the care of patients with respiratory-tract illnesses. Further, when treatment with antimicrobial agents is deemed appropriate, reliance on agents with a lower propensity to drive resistance should be encouraged.
References
Felmingham D, Grüneberg RN, the Alexander Project Group (2000) The Alexander project 1996–1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. JAC 45:191–203
Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN et al (2003) The Alexander project 1998–2002: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. JAC 52:229–246
Klugman KP (1990) Pneumococcal resistance to antibiotics. Clin Microbiol Rev 3:171–196
Beekmann SE, Heilmann KP, Richter SS, Garcia-de-Lomas J, Doern GV (2005) Antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis and group A β-hemolytic streptococci in 2002–2003: results of the multinational GRASP Surveillance Program. Int J Antimicrob Agents 25:148–156
Jones ME, Blosser-Middleton RS, Critchley IA, Karlowsky JA, Thornsberry C, Sahm DF (2003) In vitro susceptibility of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: a European multicenter study during 2000–2001. CMI 9:590–599
Doern GV, Heilmann KP, Huynh HK, Rhomberg PR, Coffman SL, Brueggemann AB (2001) Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999–2000, including a comparison of resistance rates since 1994–1995. Antimicrob Agents Chemother 45:1721–1729
Goossens H, Ferech M, Vander Stichele R, Elseviers M (2005) Outpatient antibiotic use in Europe and association with resistance: a cross national database study. Lancet 365:579–587
Garcia-Rey C, Aguilar L, Baquero F, Casal J, Dal-Re R (2002) Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. JCM 40:159–164
Bronzwaer SL, Cars O, Bücholz U (2002) European study on the relationship between antimicrobial use and antimicrobial resistance. Emerg Infect Dis 8:278–282
Melander E, Ekdahl K, Jönsson G, Mölstad S (2000) Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J 19:1172–1177
Clinical and Laboratory Standards Institute (2006) Performance standards for antimicrobial susceptibility testing: approved standard and informational supplement M100-S16. CLSI, Wayne, PA
Chen DK, McGeer A, de Azavedo JC, Low DE (1999) Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada, Canadian Bacterial Surveillance Network. N Engl J Med 341:233–239
World Health Organization (2004) Collaborating Centre for Drug Statistics Methodology. ATC index with DDDs. WHO, Oslo, Norway
Ferech M, Coenen S, Malhotra-Kumar S, Dvorakova K, Hendrickx E, Suetens C, Goossens H (2006) European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe. J Antimicrob Chemother 58:401–407
Vander Stichele R, Elseviers M, Ferech M et al (2004) European surveillance of antimicrobial consumption (ESAC): data collection performance and methodological approach. Br J Clin Pharmacol 58:419–428
Acknowledgement
This study was supported by a grant from Abbott Laboratories, Abbott Park, IL, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riedel, S., Beekmann, S.E., Heilmann, K.P. et al. Antimicrobial use in Europe and antimicrobial resistance in Streptococcus pneumoniae . Eur J Clin Microbiol Infect Dis 26, 485–490 (2007). https://doi.org/10.1007/s10096-007-0321-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0321-5