Abstract
Botulism in humans is caused by botulinum neurotoxins, produced in most cases by Clostridium botulinum, although other Clostridia species are implicated as well. Of the five forms of botulism in humans, three are referred to as “infective”: wound botulism, infant botulism, and adult intestinal botulism; the latter two forms are also referred to as “intestinal toxemia botulism” because the organism colonizes the lumen of the intestinal tract and produces botulinum neurotoxin in vivo. Twenty-three cases of infant botulism and three cases of adult intestinal botulism occurred in Italy between 1984 and 2005. Microbiological analyses of clinical, environmental, and food samples and analysis of clinical and epidemiological data revealed two main characteristics of intestinal toxemia botulism in Italy that are not common in cases in other countries: the isolation of a strain of C. butyricum that produced botulinum neurotoxin type E in 6 of 26 cases, including two cases of adult intestinal toxemia botulism, and the onset of botulism in these cases with concomitant severe gastrointestinal symptomatology. This report summarizes the microbiological, clinical, and epidemiological data of all cases of intestinal toxemia botulism that have occurred in Italy in the period 1984–2005.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are five forms of botulism in humans, all caused by botulinum neurotoxins (BoNTs), which exert their activity by blocking the release of acetylcholine at somatic and autonomic nerve terminals. Seven antigenically distinct toxin types (A–G) have been identified [1]. The disease is generally characterized by the onset of an acute weakness of the muscles innervated by cranial nerves, with progressive symmetric descending paralysis.
In addition to foodborne and iatrogenic botulism caused by ingested or improperly injected BoNT, there are three infective forms, caused by BoNT produced in vivo by neurotoxigenic microorganisms. These include wound botulism, in which the organism grows in a wound; infant botulism, in which the organism colonizes the intestinal tract of infants (first reported in 1976 [2, 3]); and adult intestinal botulism (also referred to as “hidden botulism” or “other botulism”), first reported and well documented in 1986 [4], in which the organism colonizes the intestinal tract of children and adults. The latter two forms also are referred to as “intestinal toxemia botulism” [5]. Epidemiological data demonstrate that, outside of the USA, very few cases of intestinal toxemia botulism have been reported worldwide, and even then, only in certain countries.
BoNTs are classically produced by Clostridium botulinum; however, since 1979 other BoNT-producing species have been identified. These include C. baratii, which produces BoNT type F (BoNT/F) and has been isolated from ten cases of intestinal toxemia botulism in the USA [6], one case of intestinal toxemia botulism in Hungary [7], and one case of foodborne botulism in California [8], and C. butyricum, which produces BoNT type E (BoNT/E) and has been isolated from six cases of intestinal toxemia botulism in Italy [9–12], one case of infant botulism in Japan [13], and three outbreaks of foodborne botulism in China [14], India [15], and Italy [11]. In addition, a retrospective analysis in China identified neurotoxigenic C. butyricum in two previous outbreaks of type E foodborne botulism that occurred in 1973 and 1983 [16].
To the best of our knowledge, 57 cases of infant botulism have been reported in Europe since the first case was identified in 1978 [17]. These included one case in the Czech Republic, two in Denmark, one in Finland, one in France, four in Germany, two in Hungary, 23 in Italy, three in the Netherlands, four in Norway, nine in Spain, one in Sweden, one in Switzerland, and five in the UK [18–22]. Adult intestinal botulism has only been reported in the USA [6, 23, 24], Italy [10], and Japan [25]. After the USA and Argentina [26], Italy has the third highest number of cases of intestinal toxemia botulism.
This report summarizes the cases of intestinal toxemia botulism documented in Italy from 1984 to 2005, describing the clinical manifestations as well as the environmental and laboratory data. Some of these cases have been published previously as case reports [9–12, 27–31], in which more characteristics are detailed.
Materials and methods
Surveillance system and source of specimens
In Italy, botulism has been subject to mandatory notification since 1975 and to immediate reporting since 1990. Any suspected case of botulism is to be reported immediately to the National Botulism Surveillance System [32]. The National Reference Centre for Botulism (NRCB) at the Istituto Superiore di Sanità performs active surveillance of botulism and the laboratory confirmation of suspected cases. The physician reports the suspected case to the local health authority, to the Ministry of Health, and to the NRCB and submits biological and food samples for laboratory confirmation. Positive and negative results are communicated to the physician, the health authorities, and the Ministry of Health. The Ministry of Health reports the confirmed cases to the National Institute of Statistics (ISTAT). These data are published yearly, by month and region.
At the time a case of infant botulism is suspected, a specific report form is sent to the NRCB, together with biological samples for laboratory confirmation. This form is used to interview parents and physicians about the patient’s history, clinical symptoms, and risk factors and to verify the hospital medical records. In addition, other data were also collected from telephone interviews conducted with the patient’s family and physician at the time of a suspected diagnosis or during the hospital stay to obtain extensive epidemiological information. The case definitions of infant botulism and adult intestinal botulism are those given by the US Centers for Disease Control and Prevention (CDC) [33]. The laboratory diagnosis of botulism was based on the detection of BoNT in stool or serum or the isolation of BoNT-producing clostridia from stool. In addition to C. botulinum, other BoNT-producing clostridia, such as C. butyricum, were considered in the criteria for laboratory diagnosis.
For the purpose of case classification, infant botulism was considered a clinically compatible case that was laboratory confirmed in a child below 1 year of age, and adult intestinal botulism a clinically compatible case that was laboratory confirmed in a patient ≥1 year of age who had no wounds and no history of having ingested suspect food. The clinical picture of infant botulism includes constipation, difficulty in swallowing, a weak cry, poor sucking ability, and generalized hypotonicity. In adults, the clinical manifestations of intestinal botulism comprise the classical triad of the foodborne form: a symmetrical, descending, flaccid paralysis; a clear sensorium; and an absence of fever.
Sera samples were analyzed for BoNTs, and stool samples were tested for BoNTs and the spores of neurotoxigenic clostridia. Most often, it was not possible to obtain a stool sample due to constipation; therefore, fecal swabs (more than 1 swab for each sampling) were analyzed for neurotoxigenic spores. In addition, food and environmental samples were analyzed to find the spore vehicle. Environmental samples (soil from potted plants; dust from vacuum cleaner bags; surface swabs from the infant’s room, toys, and pacifiers) were taken during visits to the homes of six infants. In 16 cases, the patient’s partially consumed food (honey, tea infusion, milk formula, and cookies) was searched for spores.
Laboratory methods
Detection of BoNTs and BoNT-producing clostridia in biological, environmental, and food samples was performed according to standard methods reported elsewhere [34], with some modifications. In the isolation step of the neurotoxigenic strain on egg yolk agar, lipase-negative colonies (C. butyricum and C. baratii) as well as lipase-positive colonies (C. botulinum) were examined.
The calculation of minimal lethal dose (MLD) of toxin was performed according to the AOAC method [35]. The spore count of neurotoxigenic organisms was performed using the five-tube most probable number (MPN) technique, with TPGY broth as culture medium. The stool samples were heated at 70°C for 10 min before inoculation into TPGY broth and the tubes incubated under anaerobic conditions for 5 days at 37°C. Mouse bioassay was used to detect toxin in the positive cultures.
Neurotoxigenic strains were identified using methods described elsewhere [10].
Results
Cases of infant and adult intestinal botulism were confirmed by isolation of C. botulinum or neurotoxigenic C. butyricum in stool samples of patients with a clinical illness of neuromuscular weakness consistent with the diagnosis of botulism. Additionally, BoNT was detected in stool samples from 11 infants and two adults and in serum samples from two infants and one adult (Tables 1 and 2).
Infant botulism
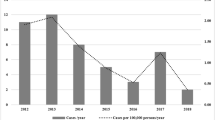
The first case of infant botulism in Italy was diagnosed in 1984 [9]. By 2005, a total of 23 cases had been reported (annual incidence 0.20/100,000 live births); 16 were caused by C. botulinum type B proteolytic, four by C. butyricum type E, and three by C. botulinum type A. In this study, reference was generally made to 22 cases, given that one case was not investigated by the NRCB and little information was available. All but four cases were reported from central and northern Italy (ten in Rome, i.e. central Italy).
All 22 infants lived in urban areas and with a good standard of living. Most parents reported that they had maintained good hygienic conditions. In the three most recent cases, renovation work was being done at the homes (information was not available for the earlier cases).
Thirteen infants were females. Seventeen infants were delivered by natural birth. The mean birth weight was 3,433 g (median, 3,410 g). All infants had been in good health before the onset of illness and had developed normally. All infants were hospitalized. The mean age at hospitalization was 14.4 weeks (median, 12 weeks); 13 were 12 weeks old or younger (the youngest was 4 weeks old), and the eldest two were 32 weeks old. The mean hospital stay was 25.9 days (median, 25.0 days). Fourteen infants (64%) had been breast-fed exclusively, two (9%) had been formula-fed exclusively, and six (27%) had been both breast- and formula-fed. Seven infants (32%) were being weaned before the onset of symptoms. No difference in the mean age at illness onset was noted between breast-fed and formula-fed babies. Sixteen infants (73%) had been given honey (in most cases, placed on the pacifier) before the onset of symptoms.
In most cases, the clinical illness was mild (Table 3). The first sign was constipation, followed by hypotonia, poor sucking ability, and weak cry. Respiratory failure was observed in eight infants and dyspnea in five. The clinical outcome was extremely serious for three patients. One of them, from whom C. botulinum type B was isolated, was diagnosed after having died suddenly [27]. Another patient (with C. butyricum type E) had severe neurological symptomatology. This patient also underwent surgery for ileocecal intestinal invagination, which revealed a massive Meckel’s diverticulum and abundant serous fluid; 1 day after surgery, the patient lapsed into a brief coma. The third patient (with C. botulinum type A) had concomitant viral intestinal infection and also was briefly comatose [28].
The four infants with C. butyricum type E had severe concomitant gastroenteric symptoms; in the most recently diagnosed case, C. difficile and its toxin were detected in feces [29]. This patient also had a biphasic course of disease. The infant was hospitalized for only 7 days and was discharged in a good state of health. The search of spores of C. butyricum in fecal samples was positive up to 45 days after discharge and negative in a single check after 65 days. Only after 1 year were we notified by the hospital that, 80 days after being discharged, the infant was readmitted for 7 days with mild neurological symptomatology, but no fecal samples were taken for analysis at the time.
Broad-spectrum antibiotics were used in 12 cases (Table 1) for the following reasons: as postsurgical therapy, as treatment of urinary tract infections, or because severe infection was suspected initially. Antibiotics used included ampicillin, oxacillin, ceftazidime, cephalosporin, ceftriaxone, netilmicin, and amoxicillin. Electromyography was performed as electrodiagnosis in 15 of the 22 cases; a positive response (>20% incremental response to repetitive stimulation at 20–50 Hz) was observed in 13 cases. Assisted ventilation was required only for the eight infants with respiratory failure. A nasal gastric probe was used to feed ten of the infants. No infants received specific antibotulinic treatment.
Botulism was laboratory confirmed in all cases (Table 1); thus, the serotype of toxin was determined and the neurotoxigenic strain was isolated. Sixteen of the 23 (70%) cases had type B infant botulism, four (17%) had type E, and three (13%) had type A. The serum samples of 12 patients were analyzed for BoNTs: one was positive for BoNT/E, another was positive for BoNT/B, and the others were negative. The fecal samples of 15 patients were investigated for the presence of BoNTs (due to constipation, only rectal swabs were obtained from the others): six were positive for BoNT/B, three for BoNT/E, and two for BoNT/A. The remaining four samples were negative. The mouse MLD of BoNT per gram of feces was determined for six patients: 40 MLD/g and 750 MLD/g for two patients with type E botulism; 160 MLD/g, 250 MLD/g, and 800 MLD/g for three patients with type B botulism; and 1,600 MLD/g for one patient with type A botulism. The fecal samples of all 23 patients were investigated for the presence of neurotoxigenic spores: C. botulinum type B spores were detected in 16 cases, BoNT/E-producing C. butyricum spores in four cases, and C. botulinum type A spores in three cases. The spore count was determined only for the nine patients from whom sufficient fecal material was collected. Four of the nine were treated with antibiotics, and in three patients, fecal samples were collected after the start of treatment. Spore counts ranged from 2.4 × 102 to 5.0 × 106 spores per gram of feces.
To evaluate the persistence of spores in the intestine, fecal samples from 15 patients were examined every 2–4 days. The spores persisted for a mean of 40.1 days from hospitalization (median 40.0 ± 20.1 days). The persistence of BoNTs in fecal samples was evaluated in five patients, and the mean length of persistence was 42.6 days after admission to the hospital (median 35.0 ± 17.0 days).
Food substances and other environmental samples from the infants’ rooms were analyzed for spores, and all samples were negative except for some honey samples (Table 4). Honey samples were analyzed in 14 cases. Botulinal spores were isolated from five of these samples, but only in one sample was the same type of the neurotoxigenic strain (C. botulinum type B) isolated from feces of the patient [30]. In fact, in one case, C. botulinum type A was isolated from the honey, whereas BoNT/E-producing C. butyricum was isolated from the feces [12]. In one case, C. botulinum type B was isolated from the honey and C. botulinum type A from the feces [28]; in another two cases, C. botulinum type A was isolated from the honey and C. botulinum type B from the feces. In the other cases, only non-neurotoxigenic clostridia were detected.
Adult intestinal botulism
The main characteristics of the three cases of adult intestinal botulism reported to date in Italy are provided in Table 2. All three cases occurred in northern Italy. Two occurred in males (9 and 56 years old) and one in a female (19 years old).
The 9-year-old male was diagnosed in 1994 and the 19-year-old female in 1995. Both had similar peculiar characteristics [10] consisting of serious gastrointestinal symptoms with acute pain, and both underwent surgery for suspected appendicitis. Neither patient had fever. During surgery, both patients were found to have a Meckel’s diverticulum, which was resected along with the appendix. Both received postsurgical antibiotics. The male was treated with rifampicin, 1 g/day for 7 days, and the female with ceftazimide, 3 g/day for 15 days. The neurological symptomatology was initially mild, but it worsened rapidly after surgery; the girl appeared comatose. Both patients required mechanical ventilation. Botulism was suspected, and BoNT/E was detected in fecal samples of both patients. Investigations for BoNTs in serum samples (performed only in the male) were negative. Spores were detected in the fecal samples of both patients and were identified as a strain of BoNT/E-producing C. butyricum. The spores were quantified only in the female: 1.1 × 105/g of feces. The results of electromyography were compatible with botulism in both patients. The length of hospitalization was 25 days for the male, who was not treated with antitoxin, and 27 days for the female, who was treated with equine antitoxin.
The third case, diagnosed in 1997, occurred in a 56-year-old man who was admitted to hospital with diplopia and dysphagia, which are neurological signs of botulism, accompanied by nausea and vomiting, with no fever. The man had not consumed any suspect foods and had no wounds. Thirty days before the onset of botulism, he underwent heart surgery and received postsurgical antibiotic therapy consisting ceftriazone 2 g per day for 2 days. No neurotoxigenic spores were detected in the first fecal sample or from food samples taken from the home. About 1 month later, the neurological symptoms persisted, and additional fecal samples and a serum sample were taken. Spores of C. botulinum type A were isolated from the feces, and BoNT/A (8 MLD/ml) was detected in the serum. Adult intestinal botulism was diagnosed. Fecal samples continued to be positive for an additional 2 weeks, with spore counts of 90 spores per gram of feces in the first sample, 150 spores per gram in the second sample, and 2,000 spores per gram in the third sample. Spores were shown to have persisted in the feces for 45 days after hospital admission. The length of hospitalization was 90 days.
Discussion
Very few cases of intestinal toxemia botulism are recorded in adults because the disease occurs rarely or it is under-recognized. The incidence rates of infant botulism can be also considered underestimates, apart from rates in the USA, where infant botulism has become the most common form of human botulism recognized, with about 100 cases recorded annually. Under-reporting of infant botulism occurs for various reasons. First, the disease is more difficult to diagnose in infants, especially because there is a wide spectrum of clinical manifestations that are nonpathognomic and are not always present; moreover, the severity of the disease can vary greatly. For these reasons (and because the incidence is nonetheless quite low), pediatricians may not recognize the infection. In fact, the diagnosis is generally made by clinicians who have received specialized training in the disease or who have had previous experience with it and thereby maintain a very high index of clinical suspicion. Such is the case in the USA, where the Infant Botulism Treatment and Prevention Program is active in California. This program provides diagnostic and consultative medical services for infant botulism, investigates all cases in California, and conducts research to improve prevention as well as treatment of the disease.
In Italy, the NRCB performs laboratory confirmation of all cases of infant botulism and collects data about clinical and epidemiological aspects of the disease. From 1984 to 2005, 23 cases of intestinal botulism in infants and three cases in adults were recorded. The relatively higher frequency of the disease in Italy compared to other European countries is likely related to the existence of a national center dealing with botulism and the increased interest in the disease following the first case of laboratory-confirmed infant botulism in 1984, in which a strain of BoNT/E producing C. butyricum was isolated.
For all 26 cases of intestinal botulism, the toxin type was determined and the neurotoxigenic strains were isolated from fecal cultures and identified. Surprisingly 23% of the strains were identified as neurotoxigenic C. butyricum. This finding is probably the result of the investigations performed to detect neurotoxigenic strains, which are different from techniques employed to detect classic C. botulinum. The standard method for the screening uses isolation media containing egg yolk as a differential ingredient to detect the lipase-positive colonies of C. botulinum, whereas C. butyricum, like neurotoxigenic C. baratii isolated in USA, is lipase negative. Beginning in 1984, in order to detect the newly identified botulinal agents, our laboratory has been screening for both lipase-positive and lipase-negative microorganisms [8].
The high incidence of proteolytic C. botulinum type B and the mild symptomatology are consistent with data on outbreaks of foodborne botulism in Italy. In fact, 87% of all botulism cases in Italy are associated with BoNT/B, while the few, very serious cases of illness are always associated with BoNT/A, known to cause a more severe course [36].
The detection of BoNT in only 21.5% (1 case type A, and 2 cases type E) of sera from culture-positive cases is consistent with the experience reported by the CDC in the USA: BoNT was detected in sera in only 13.4% of the culture-positive cases tested and in only 2.3% of the type B cases [37]. The lack of detection of BoNT in 27.7% of culture-positive feces may be due to the fact that specimens were obtained late in the course of illness.
Concerning infant botulism, case findings are concentrated in a few central and northern regions of Italy. This is probably related to the increased awareness of physicians after they diagnose their very first case; subsequently, they become more sensitized to recognizing additional cases. In fact, 50% of all cases of infant botulism in Italy were diagnosed in Rome, at the Bambino Gesù Hospital, where the first case was identified in 1984.
Fourteen babies had been breast-fed exclusively and two had been fed only formula. The only case of sudden infant disease syndrome (SIDS) in an infant with botulism in Italy [27] occurred in one of the formula-fed infants. The other formula-fed infant had severe symptoms and eventually lapsed into a coma. This is consistent with the hypothesis that breast-feeding does not prevent botulism but does attenuate the symptomatology [5, 38–40]. Furthermore, only 32% of the patients had begun weaning, probably because the median age of all infants was just 12 weeks.
Regarding the transmission of neurotoxigenic spores, some authors report that only honey and household dust have been confirmed as vehicles, and their causative role in infant botulism is well established [20, 41, 42]. Honey has been previously implicated as a vehicle of botulinal spores because spores found in the feces of infants with botulism were the same type as those found in leftover honey. In our cases, residues of honey were analyzed for 14 of the 16 infants who had consumed honey. Five of the samples were positive for C. botulinum spores, yet in only one case were the spores found in the honey identified as the same type as that found in the fecal sample.
A 2002 survey conducted in Italy showed that none of the 250 mothers of newborns had ever heard of infant botulism and that 25% of them had given honey to their child. Discouraging the consumption of honey for infants under 1 year of age is currently considered the only means of preventing infant botulism. In 2002, the European Commission adopted an opinion of the Scientific Committee on veterinary measures relating to public health about honey and its microbiological hazards. In fact, at present there is no process that can be applied to remove or kill botulinum spores in honey without impairing product quality. Thus, it is recommended that effective and targeted information regarding the risks of infant botulism from the consumption of honey should be distributed, e.g. via leaflets, labeling, and advice to healthcare professionals.
Household dust is another vehicle of neurotoxigenic spores. The four most recent cases of infant botulism occurred in infants who lived in homes with a recent history of “unusual” dust conditions from renovation work (information was not available for the other cases). Even though the analyses of environmental samples in our cases have never provided positive results, dust could still represent a vehicle for spores that cannot be completely avoided, unlike those acquired through honey consumption.
With regard to predisposing factors for infant botulism, one of the patients in Italy had a concomitant enteroviral infection, which may have been a factor for the colonization with C. botulinum [28]; this is consistent with the hypothesis that enterovirus-induced modifications of mucin favor the early development of strictly anaerobic strains [43].
The mean length of hospitalization was of 3.7 weeks (median 3.6), with a mean of 5.9 weeks (median 6.4) for type A cases, a mean of 3.5 (median 3.5) for type B cases, and a mean 2.8 weeks (median 3.4) for type E cases.
Patients were not treated with specific therapy, and the use of immunotherapy for treatment of infant botulism is not planned in Italy. In fact, only recently did the Infant Botulism Treatment and Prevention Program in California, in collaboration with the US Food and Drug Administration and the CDC, produce and distribute the orphan drug human botulism immune globulin (BIG) statewide and nationwide as the first specific treatment for infant botulism [44]. The BIG, not yet licensed in Italy, can be obtained from the California Department of Health Services (CDHS) through Italy’s Medicines Agency. A recent report of the CDHS [44] shows the results of a randomized trial to evaluate the safety and efficacy of BIG in the treatment of infant botulism. Infants treated had a shorter mean hospital stay, from 5.7 weeks to 2.6 weeks. In particular, the mean duration of hospitalization dropped from 6.7 to 2.9 weeks for patients with type A illness and from 4.2 to 2.2 weeks for patients with type B illness.
Worldwide, there have been very few cases of adult intestinal botulism. Nonetheless, some predisposing factors for adult intestinal toxemia botulism caused by C. botulinum have been identified; specifically, abnormality of the gastrointestinal tract following inflammatory intestinal disease or surgery, and alterations produced by broad-spectrum antibiotics in the endogenous microflora, which act as a barrier to intestinal colonization [5]. However, of the three patients with adult intestinal botulism in Italy, only one, from whom C. botulinum was isolated, had been treated with broad-spectrum antibiotics prior to the onset of symptoms. The other two patients had none of the above-mentioned predisposing factors. However, in these patients, intestinal botulism was caused by BoNT/E-producing C. butyricum. There are no known predisposing factors for this infection, as these are the only known cases of adult intestinal botulism attributed to BoNT/E-producing C. butyricum. Interestingly, both of these patients had a Meckel’s diverticulum, which could represent a particularly suitable site for colonization [6, 10] and constitute a predisposing factor for C. butyricum botulism; in fact, a Meckel’s diverticulum was also found in one of the infants infected with C. butyricum [6, 9]. Although these patients were the only three that underwent surgery, we cannot exclude that other patients also may have had a Meckel’s diverticulum.
Given that a neurotoxigenic strain of C. butyricum was isolated in so many cases (four cases of infant botulism and two of the three cases of adult intestinal botulism), it is interesting that severe gastroenteric symptomatology was present in all six cases. The presence of C. butyricum, a recognized agent of enteritis in newborns [45–47], initially led us to suspect that it was responsible for both the botulism and the gastroenteritis. However, when testing for cytopathic effects on the first four strains, the results were negative [10]. In another case [29], C. difficile was found to be the cause of gastroenteritis. This prompted us to hypothesize that it had been responsible for this symptomatology in all of the cases, particularly in light of the fact that, especially in newborns, C. difficile can exist in the intestine without causing symptoms. This hypothesis is consistent with data from the USA, where the gastroenteric symptomatology in several cases of infant botulism was attributed to C. difficile, even though the patients were infected with C. botulinum [48]. Although studies for detecting neurotoxigenic C. butyricum in the environment have been conducted in Italy, no positive food or environmental samples [49] have ever been found.
In Italy as well as in other countries, broad-spectrum antibiotics are frequently used in the management of intestinal toxemia botulism, especially in infant botulism and usually when other infections are present or suspected. In addition to the six patients with gastroenteritis, another eight were treated with antibiotics for other infections or because of suspected infections (for example, suspected sepsis). Therefore, antibiotics should be administered with care. In fact, clostridiocidal antibiotics can rupture the cells of the botulinal agent, causing additional BoNTs to be released, and aminoglycosides can increase the effects of BoNTs [5]. With specific regard to botulism caused by C. botulinum, trimethoprim–sulfamethoxazole and nalidixic acid are recommended because they have been shown to have no effect on the botulinal agent [50]. With regard to C. butyricum botulism, the antimicrobial susceptibility testing that we performed on Italian strains and on four Chinese strains (two of human origin and two from the environment) showed that all strains were resistant to trimethoprim-sulfamethoxazole, although they did show a certain degree of sensitivity to nalidixic acid [51]. However, considering the necessity of using antibiotics to treat secondary bacterial infections, intravenous BIG could prevent these risks [44]. Taking into consideration that intravenous BIG has a half-life of 28 days in vivo, a single infusion will neutralize all botulinum toxin that may absorbed from the colon of an infant for at least 6 months.
In conclusion, the infective forms of botulism often go undetected. This highlights the need for initiatives in training physicians to recognize this infection and in increasing the public awareness about the disease and its manifestations. Furthermore, the collection of data in a centralized national database of clinical, epidemiological, and laboratory records, case by case, is useful for improving the understanding and knowledge of certain aspects of the disease, especially regarding host susceptibility.
References
Cherington M (2004) Botulism: update and review. Semin Neurol 24:155–163
Pickett J, Berg B, Chaplin E, Brunstetter-Shafer MA (1976) Syndrome of botulism in infancy: clinical and electrophysiologic study. N Engl J Med 295:770–772
Midura TF, Arnon SS (1976) Infant botulism: identification of Clostridium botulinum and its toxins in faeces. Lancet 2:934–936
Chia JK, Clark JB, Ryan CA, Pollak M (1986) Botulism in an adult associated with food-borne intestinal infection with Clostridium botulinum. N Engl J Med 315:239–240
Arnon SS (1995) Botulism as an intestinal toxemia. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL (eds) Infection of the gastrointestinal tract. Raven, New York, pp 257–271
Schechter R, Arnon SS (1999) Commentary: where Marco Polo meets Meckel: type E botulism from Clostridium butyricum. Clin Infect Dis 29:1388–1393
Trethon R, Budai J, Herendi A, Szabo V, Geczy M (1995) Botulism in infancy. Orv Hetil 136:1497–1499
Harvey SM, Sturgeon J, Dassey DE (2002) Botulism due to Clostridium baratii type F toxin. J Clin Microbiol 40:2260–2262
Aureli P, Fenicia L, Pasolini B, Gianfranceschi MV, McCroskey LM, Hatheway CL (1986) Two cases of type E infant botulism caused by neurotoxigenic Clostridium butyricum in Italy. J Infect Dis 154:207–211
Fenicia L, Franciosa G, Pourshaban M, Aureli P (1999) Intestinal toxemia botulism in two young people, caused by Clostridium butyricum type E. Clin Infect Dis 29:1381–1387
Anniballi F, Fenicia L, Franciosa G, Aureli P (2002) Influence of pH and temperature on the growth and toxin production by neurotoxigenic strains of Clostridium butyricum type E. J Food Prot 65:1267–1270
Franciosa G, Anniballi F, Fenicia L, Aureli P (1998) New recovery of neurotoxigenic Clostridium butyricum type E from a case of infant botulism. In: Abstracts of the European Clostridia Conference, Germany
Chie M, Yanagawa Y, Shibata M, Obata H, Yamada S, Itoh T (2006) Botulism-Yokio 1996–2005. In: Abstracts of the 43rd interagency botulism research coordinating committee meeting, Abstract no. 26
Meng X, Karasawa T, Zou K, Kuang X, Wang X, Lu C, Wang C, Yamakawa K, Nakamura S (1997) Characterization of a neurotoxigenic Clostridium butyricum strain isolated from the food implicated in an outbreak of food-borne type E botulism. J Clin Microbiol 35:2160–2162
Chaudhrj R, Dhawan B, Kumar D, Bhatia R, Gandhi JC, Patel RK, Purohit BC (1998) Outbreak of suspected Clostridium butyricum botulism in India. Emerg Infect Dis 4:506–507
Meng X, Yamakawa K, Zou K, Wang X, Kuang X, Lu C, Wang C, Karasawa T, Nakamura S (1999) Isolation and characterization of neurotoxigenic Clostridium butyricum from soil in China. J Med Microbiol 48:133–137
Turner HD, Brett EM, Gilbert RJ, Ghosh AC, Liebeschuetz HJ (1978) Infant botulism in England. Lancet 1:1277–1278
Neubauer M, Milacek V (1981) Infant botulism type B in central Europe. Zentalbl Bakteriol Mikrobiol Hyg [A] 250:540–547
Aureli P, Franciosa G, Fenicia L (2002) Infant botulism and honey in Europe: a commentary. Pediatr Infect Dis J 21:866–868
Nevas M, Lindstrom M, Virtanen A, Hielm S, Kuusi M, Arnon SS, Vuori E, Korkeala H (2005) Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol 43:511–513
Thomasse Y, Arends JP, van der Heide PA, Smit LM, Weerden TW, Fock JM (2005) Three infants with constipation and muscular weakness: infantile botulism. Ned Tijdschr Geneeskd 149:826–831
Johnson EA, Tepp WH, Bradshaw M, Gilbert RJ, Cook PE, McIntosh EDG (2005) Characterization of Clostridium botulinum strains associated with an infant botulism case in the United Kingdom. J Clin Microbiol 43:2602–2607
Shapiro RL, Hatheway C, Swerdlow DL (1998) Botulism in the United States: a clinical and epidemiologic review. Ann Intern Med 129:221–228
Gupta A, Sumner CJ, Castor M, Maslanka S, Sobel J (2005) Adult botulism type F in the United States, 1981–2002. Neurology 65:1694–1700
Kobayashi H, Fujisawa K, Saito Y, Kamijo M, Oshima S, Kubo M, Eto Y, Monma C, Kitamura M (2003) A botulism case of a 12-year-old girl caused by intestinal colonization of Clostridium botulinum type Ab. Jpn J Infect Dis 56:73–74
Centorbi HJ, Aliendro OE, Demo NO, Dutto R, Fernandez R, Centorbi ONP (1998) First case of infant botulism associated with honey feeding in Argentina. In: Abstracts of the Anaerobe Society of the Americas Congress on anaerobic bacteria and anaerobic infections. Abstract no. 15PP
Aureli P, Ferrini AM (1988) Identification of C. botulinum spores in a case of sudden infant death in Italy. Description of a clinical case. Minerva Pediatr 40:125–126
Fenicia L, Anniballi F, Pulitanò S, Genovese O, Polidori G, Aureli P (2004) A severe case of infant botulism caused by Clostridium botulinum type A with concomitant intestinal viral infections. Eur J Pediatr 163:501–502
Fenicia L, Da Dalt L, Anniballi F, Franciosa G, Zanconato S, Aureli P (2002) A case of infant botulism due to neurotoxigenic Clostridium butyricum type E associated with Clostridium difficile colitis. Eur J Clin Microbiol Infect Dis 21:736–738
Fenicia L, Ferrini AM, Aureli P, Pocecco M (1993) A case of infant botulism associated with honey feeding in Italy. Eur J Epidemiol 9:671–673
Aureli P, Fenicia L, Creti R, Bertini E, Vigevano F, Di Capua M, Pirozzi N (1989) Botulismo infantile in Italia. Aspetti clinici e microbiologici di 5 casi. Riv Ital Pediatr 15:442–447
Squarcione S, Prete A, Vellucci L (1999) Botulism surveillance in Italy: 1992–1996. Eur J Epidemiol 15:917–922
Centers for Disease Control and Prevention (1997) Case definitions for infectious conditions under public health surveillance. MMWR 46:1–55. http://www.cdc.gov/mmwr/PDF/RR/RR4610.pdf and http://bt.cdc.gov/Agent/Botulism/CaseDef.asp
Centers for Disease Control and Prevention (1998) Botulism in the United States, 1899–1996. Handbook for epidemiologists, clinicians, and laboratory workers. CDC, Atlanta, GA, pp 15–21
AOAC Official Methods of Analysis (2000) Clostridium botulinum and its toxins in foods. AOAC official method n. 977.26
Woodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL (1992) Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. J Infect Dis 166:1281–1286
Hatheway CL, McCroskey LM (1987) Examination of feces and serum for diagnosis of infant botulism in 336 patients. J Clin Microbiol 25:2334–2338
Arnon SS, Damus K, Thompson B, Midura TF, Chin J (1982) Protective role of human milk against sudden death from infant botulism. J Pediatr 100:568–573
Arnon SS (1984) Breast feeding and toxigenic intestinal infections: missing links in crib death? Rev Infect Dis 6(Suppl 1):193–201
Long SS, Gajewski JL, Brown LW, Gilligan PH (1985) Clinical, laboratory and environmental features of infant botulism in southeastern Pennsylvania. Pediatrics 75:935–941
Arnon SS, Midura TF, Damus K, Thompson B, Wood RM, Chin J (1979) Honey and other environmental risk factors for infant botulism. J Pediatr 94:331–336
Arnon SS, Damus K, Chin J (1981) Infant botulism: epidemiology and relation to sudden infant death syndrome. Epidemiol Rev 3:45–66
Moreau MC, Corther G, Hulle MC, Dubos F, Raibaud P (1986) Relationships between rotavirus diarrhea and intestinal microflora establishment in conventional and gnotobiotic mice. J Clin Microbiol 23:863–868
Arnon SS, Schechter R, Maslanka SE, Jewell NP, Hatheway CL (2006) Human botulism immune globulin for the treatment of infant botulism. N Engl J Med 354:462–471
Howard FM, Bradley JM, Flynn DM, Noone P, Szawatkowski M (1977) Outbreak of necrotizing enterocolitis caused by Clostridium butyricum. Lancet 2:1099–1102
Laverdiére M, Robert A, Chicoine R, Salet D, Rosenfeld R (1978) Clostridia in necrotising enterocolitis. Lancet 2:377
Smith MF, Borriello SP, Clayden GS, Casewell MW (1980) Clinical and bacteriological findings in necrotizing enterocolitis: a controlled study. J Infect 2:23–31
Schecter R, Peterson B, McGee J, Idowu G, Bradley J (1999) Clostridium difficile colitis associated with infant botulism: near-fatal case analogous to Hirschsprung’s enterocolitis. Clin Infect Dis 29:143–146
Creti R, Fenicia L, Aureli P (1990) Occurrence of Clostridium botulinum in the soil in the vicinity of Rome. Curr Microbiol 20:317–321
Swenson JM, Thornsberry L, McCroskey L, Hatheway CL, Dowell VR (1980) Susceptibility of C. botulinum to thirteen antimicrobial agents. Antimicrob Agents Chemother 18:13–19
Fenicia L, Ferrini AM, Anniballi F, Mannoni V, Aureli P (2003) Considering the antimicrobial sensitivity of the intestinal botulism agent Clostridium butyricum when treating concomitant infections. Eur J Epidemiol 18:1153–1154
Acknowledgements
We thank Stephen S. Arnon, founder and chief of the Infant Botulism Treatment and Prevention Program, California Department of Health Services, for his kind assistance in the editorial review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fenicia, L., Anniballi, F. & Aureli, P. Intestinal toxemia botulism in Italy, 1984–2005. Eur J Clin Microbiol Infect Dis 26, 385–394 (2007). https://doi.org/10.1007/s10096-007-0301-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0301-9