Abstract
In response to several isolations of methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leucocidin gene (PVL-MRSA), the present study was conducted to document the spread of infection in a small region of southeastern Germany. During a 9-month period, two healthcare-associated outbreaks with PVL-MRSA occurred, affecting 83 patients, personnel and contacts of personnel, and 34 additional cases were detected in the community. The clinical spectrum ranged from colonization to skin infection and necrotizing pneumonia. The findings represent the largest number of PVL-MRSA cases detected in Germany so far, and demonstrate the potential of this emerging pathogen to spread within the community and in healthcare institutions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, methicillin-resistant Staphylococcus aureus harboring the Panton-Valentine leucocidin gene (PVL-MRSA) was considered to be rare in Germany [1]. Worldwide, PVL-MRSA is recognized as an emerging community-acquired pathogen causing skin-related infections and necrotizing pneumonia in persons with no apparent risk factors, including children [2–4]. Transmission in hospitals has also been observed [5]. PVL-MRSA, also called community-acquired or community-associated MRSA, possesses a unique combination of pathogenicity and resistance factors. Among other virulence factors, Panton-Valentine leucocidin (lukS/F-PV), which causes lysis of granulocytes and monocytes as well as tissue necrosis, is considered to be of special importance [6]. Resistance to oxacillin is encoded by the mecA gene typically present on a mec type IV [7] or type II staphylococcal cassette chromosome (SCC).
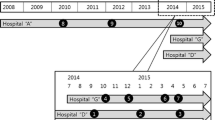
In Regensburg, the first isolate of PVL-MRSA related to this study was found in an 18-year-old woman in December 2003. In January 2004, the detection of PVL-MRSA in one nursing home resident and in one nursing home employee within a 7-day period prompted a systematic investigation of all residents and personnel in two nursing homes. The strain isolated from the patient was detected during routine hospital admission screening and that of the employee was the causative agent of community-acquired pneumonia. A second outbreak was observed in a neonatal care unit. During the time of the investigation, an unprecedented number of patients with community-acquired PVL-MRSA was also reported in the area under study. Described here are the clinical findings from 117 cases of healthcare-associated and community-acquired PVL-MRSA detected between December 2003 and August 2004.
Patients and methods
This observational study was conducted between December 2003 and August 2004 in a geographically well-defined region, measuring approximately 50 km in radius, around the city of Regensburg, in southeastern Germany. Six laboratories (Institute for Medical Microbiology, University Regensburg; Microbiology Laboratory of the St. Elisabeth-Krankenhaus, Straubing; Microbiology Laboratory of the Klinikum Fürth, Fürth; and three microbiology laboratories serving outpatients) participated in the study by testing for PVL-MRSA in screening samples obtained during two outbreaks and in routine samples obtained from patients suspected of having infection.
Patient characteristics and clinical data were obtained using a standardized questionnaire. On the basis of epidemiological data, patients with PVL-MRSA were classified as hospital-acquired or community-acquired cases. A case was considered hospital-acquired if the patient was (i) staying or working in a healthcare institution during the time of sampling, (ii) identified by screening during the investigation of outbreak I or II, or (iii) a close contact of a person identified by screening. In outbreak I, screening samples were obtained from all residents (anterior nares, groin, wounds if applicable) and personnel (anterior nares) in two nursing homes. In outbreak II, samples obtained from all patients (anterior nares, ear, groin, feces, wounds if applicable) and personnel (anterior nares) in a neonatal care unit were screened. A case was considered community-acquired, if the patient had not been treated as an inpatient within the last 6 months, and was not affiliated with a hospital, nursing home, or any other healthcare institution. All patients were encouraged to report close private contacts with symptoms or a history of recurrent skin infection, and close contacts were screened on a voluntary basis.
Routine clinical specimens were plated directly, without pre-enrichment, onto non-selective and selective media (Orsab agar; Oxoid, Basingstoke, UK). For screening, swabs were moistened with sterile saline before use, and a pre-enrichment step (thioglycolate broth, Oxoid) was included.
Susceptibility testing was performed according to the guidelines of the National Committee for Clinical Laboratory Standards [8], and resistance to fusidic acid was tested according to previously described procedures [9]. Results were confirmed by simultaneous real-time PCR-based detection of the S. aureus-specific marker nuc and the mecA gene [10]. LukS/F-PV and hlyA genes were amplified by block-cycler PCR as described previously [3]. Typing of selected isolates was performed using SmaI-macrorestriction and multilocus sequence typing (http://www.MLST.net) [11].
Results and discussion
During a 9-month period, from December 2003 to September 2004, we observed 117 cases of PVL-MRSA colonization or infection in a small region of southeastern Germany (within a radius of 50 km around Regensburg). Eighty-three of the cases were found in two healthcare-associated outbreaks. Outbreak I occurred in a cluster of 10 healthcare institutions (2 hospitals, 5 nursing homes, 1 home for disabled persons, 1 hemodialysis outpatient clinic, and 1 patient transport service) in two closely positioned cities; it involved 52 patients and nursing home residents as well as 21 personnel and 2 private contact persons. This outbreak prompted screening of all residents (n=394) and personnel (n=192) in two nursing homes and revealed a PVL-MRSA carrier rate of 9.1% in residents and 9.7% in personnel (the carrier rates for MRSA isolates susceptible to fusidic acid and negative for the lukS/F-PV genes were 3.5% and 1%, respectively). Outbreak II occurred in a neonatal care unit and involved 5 of 20 patients and 3 of 131 personnel. During the same time period, 34 cases of community-acquired PVL-MRSA were found. Community-acquired cases of PVL-MRSA were identified, in part, due to referral of patients with recurrent subcutaneous abscesses after the first case of necrotizing pneumonia due to PVL-MRSA was reported in a local newspaper.
The demographic and clinical details of all cases are given in Table 1. Community-acquired cases had higher rates of clinical disease than the cases identified by screening in the healthcare-associated outbreaks. When patients and personnel carrying PVL-MRSA from outbreaks I and II were compared, the rate of clinical disease was 28.1% for patients and 16.6% for personnel. Spontaneous abscesses occurred at similar rates in both groups (12.3% in patients, 16.6% in personnel). Nearly all 117 PVL-MRSA isolates showed a uniform antimicrobial susceptibility pattern: All isolates were resistant to oxacillin and resistant or intermediately susceptible to fusidic acid (1 was susceptible) but susceptible to macrolides, tetracyclines (3 resistant), or quinolones (1 resistant). All were positive for the lukS/F-PV genes. Results of pulsed-field gel electrophoresis and multilocus sequence typing were in accordance with epidemiological data from the patients and suggested a high degree of relatedness within the two groups of isolates from the two healthcare-associated outbreaks (outbreak I: ST22, n=33/33; outbreak II: ST80, n=8/8), whereas a variety of multilocus sequence types was found among the community-acquired cases (ST80, n=10/21; ST22, n=8/21; ST30, n=2/21; ST8, n=1/21).
Worldwide, PVL-MRSA is recognized as an emerging pathogen [12, 13]. The results of our study in southeastern Germany are new and noteworthy in several respects. The high number of PVL-MRSA isolates we found within a small geographical region (n=117) during a 9-month period far exceeds the previously reported and confirmed total number of 23 PVL-MRSA isolates found in Germany until the end of 2003 [14]. This raises the question as to what extent PVL-MRSA is currently underreported in Germany, especially in southeastern Germany.
In our investigation, PVL-MRSA isolates were detected in two distinct settings, community and healthcare. The two outbreaks in healthcare settings demonstrate the potential of PVL-MRSA to spread as a healthcare-associated pathogen. The similar rates of cutaneous infection found by screening patients and personnel in outbreaks I and II support the concept that no special risk factors are needed in order for an individual to contract a skin infection due to S. aureus carrying the PVL gene. The high rate of clinical manifestations we observed among the community-acquired cases is most likely due to detection bias. In our study, cases of community-acquired PVL-MRSA were not retrieved systematically, thereby making it impossible to determine whether PVL-MRSA has been increasing in our region recently. We cannot exclude that PVL-MRSA may have been prevalent in the region before and gone undetected. This is especially true for community-acquired cases, which are rarely subjected to microbiological testing.
Several aspects of PVL-MRSA might become a severe problem in the future. Compared to common S. aureus strains susceptible to oxacillin, PVL-MRSA are unique in terms of their virulence, antibiotic resistance, and tendency to spread. PVL-MRSA can cause significant infections in people with and without risk factors, and treatment options are limited due to the pathogen’s resistance to all beta-lactam antibiotics. Obviously, selective pressure by antibiotics is not necessary for successful dissemination of PVL-MRSA in the community, and it easily occurs in the healthcare setting. In response to outbreak I, appropriate steps for the diagnosis, hygiene, and therapy of patients and personnel were defined in collaboration with the public health authorities. Further studies of the epidemiology of PVL-MRSA in the community and in healthcare institutions in southern Germany are warranted.
References
Witte W, Cuny C, Strommenger B, Braulke C, Heuck D (2004) Emergence of a new community acquired MRSA strain in Germany. Euro Surveill 9:1–2
Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J (2003) Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9:978–984
Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, Vandenesch F, Etienne J (1999) Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis 29:1128–1132
Anonymous (1999) Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus: Minnesota and North Dakota, 1997–1999. MMWR 48:707–710
Saiman L, O’Keefe M, Graham III PL, Wu F, Said-Salim B, Kreiswirth B, LaSala A, Schlievert PM, Della-Latta P (2003) Hospital transmission of community-acquired methicillin-resistant Staphylococcus aureus among postpartum women. Clin Infect Dis 37:1313–1319
Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K (2002) Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359:1819–1827
Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, Daum RS, Hiramatsu K (2002) Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother 46:1147–1152
National Committee for Clinical Laboratory Standards (1999) Performance standards for antimicrobial disk susceptibility testing. Ninth informational supplement. M100-S9. NCCLS, Villanova, PA
Skov R, Frimodt-Moller N, Espersen F (2001) Correlation of MIC methods and tentative interpretive criteria for disk diffusion susceptibility testing using NCCLS methodology for fusidic acid. Diagn Microbiol Infect Dis 40:111–116
Reischl U, Linde HJ, Metz M, Leppmeier B, Lehn N (2000) Rapid identification of methicillin-resistant Staphylococcus aureus and simultaneous species confirmation using real-time fluorescence PCR. J Clin Microbiol 38:2429–2433
Robinson DA, Enright MC (2004) Multilocus sequence typing and the evolution of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 10:92–97
Salgado CD, Farr BM, Calfee DP (2003) Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 36:131–139
Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O’Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K (2002) Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol 40:4289–4294
Witte W (2004) Community acquired MRSA weltweit und in Deutschland. Epidemiologisches Bulletin 5:33–37
Acknowledgments
We thank E. Kleingütl (Institut für Medizinische Mikrobiologie, Universität Regensburg, Regensburg, Germany) and D. Döring (Altenheim Pro-Seniore, Straubing, Germany) for assistance in the screening of outbreak I. We thank Dr. med. F. Buchwald (synlab, Weiden, Germany), Dr. med. J. Huber (Klinikum Deggendorf, Deggendorf, Germany) and Dr. med. J. Matthes (Miclab GmbH, Neuötting, Germany) for provision of isolates. We thank M. Werkmann (Mikrobiologisches Labor, Klinikum Straubing, Germany) for laboratory assistance in the screening of outbreak I
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linde, H., Wagenlehner, F., Strommenger, B. et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur J Clin Microbiol Infect Dis 24, 419–422 (2005). https://doi.org/10.1007/s10096-005-1341-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-005-1341-7