Abstract
Background
Fingolimod is an oral daily treatment for relapsing remitting multiple sclerosis (RRMS). A decrease in lymphocytes count is a common side effect, whereby clinicians occasionally propose a reduced dose rather than its discontinuation. However, current data on the effectiveness of these regimens are scarce and contradictory. Our objective was to investigate if the fingolimod effectiveness is maintained with reduction in dosing frequency.
Methods
Retrospective and observational study of RRMS patients taking fingolimod-nondaily (FTY-ND) for at least 6 months. Propensity score–based matching was performed to select patients taking daily dose (FTY-ED) with comparable baseline characteristics: age, sex, disease duration, annualized relapse rate (ARR), and expanded disability status scale (EDSS). Afterwards, clinical and laboratorial assessment was evaluated in both groups.
Results
Thirty-six patients were included in each group (FTY-ED vs. FTY-ND). Decrease in lymphocytes count was the main reason for switching to FTY-ND (88.9%). Previous treatment with natalizumab was inversely associated with risk of reducing dose (OR 0.253, 95%CI = 0.08–0.807, p = 0.016). There were no significant differences in clinical disease activity between patients FTY-ED vs. FTY-ND: mean ARR 0.4 vs. 0.3 (p = 0.247), median EDSS 2.0 vs. 2.0 (p = 0.687), and proportion of patients with EDSS increase 8.3% vs. 13.9% (p = 0.453). FTY-ND was overall well tolerated and was associated with an increase in the mean lymphocytes count (362 ± 103 cells/mm3 to 541 ± 183 cells/mm3, p < 0.001).
Conclusion
These data suggest that the effectiveness of FTY is maintained despite the reduction of the dose, minimizing the most common adverse events. These findings warrant further confirmation, ideally with randomized clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is the most common inflammatory, demyelinating disorder of the central nervous system (CNS) [1] and the most common nontraumatic cause of neurologic disability in young adults [2]. Fingolimod (FTY) was the first approved immunomodulatory oral treatment with demonstrated efficacy and safety in relapsing remitting multiple sclerosis (RRMS) [3,4,5].
FTY is efficiently absorbed regardless of food intake, and its oral bioavailability is high (> 90%) [3, 4]. The slow absorption (maximal plasma concentration after 12–16 h) and the prolonged half-life (6–9 days) contribute to its stable concentration over time [3, 4]. It reaches steady-state concentrations after 1–2 months during daily intake [3,4,5]. FTY shows high plasma protein binding (> 99.7%), mainly to albumin [5].
In 2010, Food and Drug Administration and European Medicines Agency granted approval of FTY 0.5 mg/day as single disease-modifying therapy (DMT) in highly active RRMS. Key data were derived from 2 international main phase III, double-blind and randomized trials (TRANSFORMS and FREEDOMS) performed over 12 and 24 months, respectively, in these patients [1]. A recent meta-analysis evaluating the efficacy and safety of FTY in RRMS, including 10 studies, concluded a significantly lower annualized relapse rate (ARR) (mean difference − 0.22) than placebo, a favorable performance on magnetic resonance imaging outcomes and an improvement of the quality of life [6].
Overall, the FTY-approved dose of 0.5/day was the lowest tested dose, and it has shown to have a favorable safety profile and good tolerability.
In clinical practice, to address these issues of persistent severe lymphopenia or elevated liver enzymes, many neurologists propose a reduced dose of FTY rather than its discontinuation, given its long half-life and stable plasma concentration. However, current data on the effectiveness of less frequent, non-approved dosing regimens are scarce and contradictory [4, 7].
Therefore, this study aimed to investigate if the FTY effectiveness is maintained with reduction of dosing frequency.
Methods
Study design
This was a unicentric, retrospective, and observational study conducted at a tertiary Multiple Sclerosis Center in Portugal. We aimed to characterize multiple sclerosis patients who switched from FTY-everyday (FTY-ED) to FTY-nondaily (FTY-ND) in real-world clinical practice and compare the efficacy and safety of FTY-ED and FTY-ND. The study was approved by the local Ethics Committee.
Study population
We identified all RRMS adult patients followed at our Multiple Sclerosis Center taking FTY-ND between December 2012 and December 2019, for at least 6 months, who were treated originally with the approved dose. The decision to switch to FTY-ND was made by the treating neurologist based on clinical criteria. Propensity score–based matching was performed to select similar number of patients taking FTY-ED with comparable clinical baseline characteristics: age, sex, disease duration, ARR, and expanded disability status scale (EDSS).
Clinical and laboratorial assessment
Clinical relapses were defined as new neurologic symptoms or reactivation of preexisting neurological deficits lasting more than 24 h that were judged by the assistant neurologist to represent new MS activity. ARR was calculated based on the number of relapses divided by the length of follow-up, measured in years. Disability was determined using the EDSS. Pre-treatment analytic blood screening was performed in all patients according to the guideline’s recommendations. Complete blood cell count and liver function were repeated every 3 months in the first year of treatment (FTY-ED and FTY-ND) and then every 6 months. Grades of lymphopenia were defined according the common terminology criteria for AEs: grade 1, the lower limit of normal to 800 cells/mm3; grade 2, 799–500 cells/mm3; grade 3, 499–200 cells/mm3; and grade 4, below 199 cells/mm3 [8]. Reference values of our laboratory for liver function are aspartate aminotransferase (AST) < 31 U/L and alanine aminotransferase (ALT) < 34 U/L.
Statistical analysis
Baseline characteristics are presented as frequency and percentage for dichotomous variables and mean ± standard deviation (SD) for quantitative variables. Paired t tests were used to compare clinical activity of disease (ARR, EDSS, and EDSS increasing) in FTY-ED vs. FTY-ND. Univariable and multivariable logistic regression models were used to identify variables that could predict the risk of using FTY-ND.
Patients were matched on their propensity for receiving FTY-ED versus FTY-ND. The propensity score was based on a multivariable logistic regression model with treatment allocation as the outcome variable and the demographic and clinical variables at the treatment baseline as the independent variables. These comprised age, sex, disease duration, ARR, and EDSS. Patients were then matched in a 1:1 ratio using nearest neighbor matching within a caliper of 0.2 SD of the propensity score. Patients who switched or discontinued treatment were censored.
Statistical significance was set for p < 0.05. All the statistical analyses were made in IBM SPSS Statistics software, version 25.
Results
Baseline characteristics
A total of 72 patients with RRMS were enrolled, 36 under FTY-ND and 36 with FTY-ED. Baseline characteristics were similar between both groups (Table 1).
By the time of FTY initiation, most patients had previously received other DMT, in most cases beta-interferon or glatiramer acetate (n = 41, 56.9%), 19 were previously treated with natalizumab (26.4%), and only 7 were naïve patients (9.7%).
Patients were on FTY-approved dose during 18.5 ± 16.01 months before switching to reduced dose. Seventy-five percent of patients were on FTY-ND more than 1 year. Persistent reduction of lymphocytes count was the main reason for switching from FTY-ED to FTY-ND (n = 32, 88.9%). The other patients presented persistent liver enzymes elevations (n = 4, 11.1%). All patients who were switched to FTY-ND had grades 3 or 4 of lymphopenia. The mean lymphocytes count before reducing dose was 362 ± 103 cells/mm3.
Regarding liver enzymes evaluation, mean levels of AST was 104.25 ± 78.76 U/L, and ALT was 107.5 ± 63.61 U/L before reducing dose.
Considering previous treatments, the use of natalizumab before FTY was inversely associated with the probability of using a reduced dose (OR 0.253, 95%CI = 0.08–0.807, p = 0.016), while age, disease duration, lymphocytes count before FTY, baseline EDSS, and ARR were not significant predictors (Table 2).
Efficacy
There were no significant differences in clinical disease activity between patients taking daily dose compared with reduced dose: the mean ARR was 0.4 vs. 0.3 (p = 0.247), and the median EDSS was 2.0 vs. 2.0 (p = 0.687), respectively (Fig. 1). Overall, patients FTY-ND had, on average, 0.41 relapses (± 0.73) during the follow-up time.
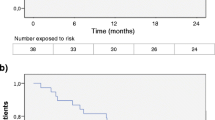
Disability progression measured by EDSS increasing occurred in 8.3% of patients FTY-ED and in 13.9% of FTY-ND, although there were no statistical differences (p = 0.453) (Fig. 2). A total of 7 patients interrupted FTY-ND during the follow-up: 1 due to inefficacy and 6 patients returned to daily dose because there was a recovery in lymphocytes count (n = 5) and of the liver enzymes levels (n = 1). The other patients continued in the alternative scheme for 23.07 ± 8.04 months.
Safety
FTY-ND was overall well tolerated and was associated with a rise in the mean lymphocytes count from 362 ± 103 cells/mm3 to 541 ± 183 cells/mm3 after reducing dose (p < 0.001). In patients who changed dose due to an increase of liver enzymes, there was a significant improvement after dose reduction, with ALT decreasing to 39.0 ± 13.69 U/L (p = 0.001) and AST dropping to 28.25 ± 8.46 U/L (p = 0.007).
No serious AE occurred during the FTY-ND regimen.
Discussion
The results obtained in our study suggest that the effectiveness of FTY was preserved despite the reduction of the dose.
The main reasons for switching the daily dose was a decrease on lymphocytes count or an increase on liver enzymes. Actually, in the FTY clinical trials, a lymphocytes count decreasing under 200 cells/mm3 was a common event during FTY-ED treatment, occurring in 15–20% of the patients and represented a trial criteria for treatment discontinuation [4]. Furthermore, in the pivotal trials, 8% of patients receiving the FTY-approved dose of 0.5 mg/day had liver enzymes elevated at least 3 times the upper limit of normal (ULN), which occurred within the first 3–4 months of treatment and returned to the normal range within 2 months of treatment discontinuation [1].
In our sample, during the follow-up, the strategy of switching to nondaily dose allowed a significant improvement of lymphocytes count above 500 cells/mm3 and a reduction on liver enzymes levels.
The strategy of FTY-ND seems to be as effective as FTY-ED and well tolerated, since there were no significant differences observed in ARR or in EDSS progression. Similar results were found in a multicenter and observational study published, supporting the treatment scheme of reduced dose in patients with good disease control but lymphopenia while on daily dose [7]. However, in a case series of 8 patients [3] and in an Italian-Swiss retrospective study [4], an increased risk of MS activity in patients treated with reduced dose of FTY was observed. In this last cohort, there was a higher risk of disease reactivation in younger patients and who were previously treated with natalizumab, whereby the authors recommend caution to reduce dose in these population [4].
Our results suggest that patients under a reduced dose of FTY may be able to achieve an adequate reduction of lymphocytes recirculation to the CNS and therefore effectively prevent disease activity [7]. Moreover, there are some studies indicating that lymphocyte count in peripheral blood is not associated with the level of clinical response to treatment [9,10,11].
We also found that patients previously treated with natalizumab presented lower probability of needing a reduced dose of FTY afterwards. A trend for an association between baseline ARR and baseline EDSS and risk of being switched from FTY-ED to FTY-ND because of lymphopenia was also observed. Other associations had been published in previous studies as female gender, lower weight, and lower lymphocytes count at baseline. [4] These can be factors to consider in selection of candidates to FTY treatment, although in our cohort disease activity with FTY-ND was no different to FTY-ED.
There are several limitations to the present study. This is a small sample and unicentric study which reduces the statistical power of the analyses. The reduced dose regimens (every other day or 5 days/week) were not standardized and could be a bias in the analyses of the results. Also, we did not have radiologic assessment data available in many patients, since a significant number of them performed magnetic resonance imaging out of our center, with different protocols making comparative studies unfeasible, and because of that radiological activity evaluation was not performed.
In conclusion, if our results are confirmed, FTY-ND regimen may be considered a clinical strategy for patients who are stable and achieved remission of the disease activity with FTY-ED, allowing to minimize the most common adverse events and therefore enhance patient safety. These findings warrant further confirmation, ideally with randomized clinical trials to establish the minimum effective dose for FTY.
Data availability
Not applicable.
Abbreviations
- AE:
-
Adverse events
- ALT:
-
Alanine aminotransferase
- ARR:
-
Annualized relapse rate
- AST:
-
Aspartate aminotransferase
- CNS:
-
Central nervous system
- DMT:
-
Disease-modifying therapy
- EDSS:
-
Expanded disability status scale
- FTY:
-
Fingolimod
- FTY-ED:
-
Fingolimod-everyday
- FTY-ND:
-
Fingolimod-nondaily
- MS:
-
Multiple sclerosis
- RRMS:
-
Relapsing remitting multiple sclerosis
- SD:
-
Standard deviation
- ULN:
-
Upper limit of normal
References
Singer B, Ross AP, Tobias K (2011) Oral fingolimod for the treatment of patients with relapsing forms of multiple sclerosis. Int J Clin Pr 65(8):887–895
LM L, Tramacere I, Firwana B, Pacchetti I, Palumbo R, Filippini G (2016) Fingolimod for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev 4:CD009371
Yamout BI, Zeineddine MM, Sawaya RA, Khoury SJ (2015) Safety and efficacy of reduced fingolimod dosage treatment. J Neuroimmunol 285:13–15
Zecca C, Merlini A, Disanto G, Rodegher M, Panicari L, Anita M et al (2018) Half-dose fingolimod for treating relapsing-remitting multiple sclerosis: observational study. Mult Scler 24(2):167–174
Huwiler A, Zangemeister-wittke U (2018) The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol Ther 185:34–39
Yang T, Tian X, Chen CY, Ma LY, Zhou S, Li M, Wu Y, Zhou Y, Cui YM (2020) The efficacy and safety of fingolimod in patients with relapsing multiple sclerosis: A meta-analysis. Br J Clin Pharmacol 86(4):637–645
Longbrake EE, Kantor D, Pawate S (2018) Effectiveness of alternative dose fingolimod for multiple sclerosis. Neurol Clin Pr 8(2):102–107
Ohtani R, Mori M, Uchida T, Uzawa A, Masuda H, Liu J (2018) Risk factors for fingolimod-induced lymphopenia in multiple sclerosis. Mult Scler J Exp Transl Clin 4(1):2055217318759692
Jeffery DR, Rammohan KW, Hawker K, Fox E, Jeffery DR, Rammohan KW et al (2016) Fingolimod: a review of its mode of action in the context of its efficacy and safety profile in relapsing forms of multiple sclerosis. Expert Rev Neurother 16(1):31–44
Dadalti Y, Spelman T, Boz C, Alroughani R, Lugaresi A, Vucic S et al (2018) Lymphocyte count in peripheral blood is not associated with the level of clinical response to treatment with fingolimod. Mult Scler Relat Disord 19:105–108
Bruschi N, Boffa G, Cellerino M et al (2019) Lymphopenia is not associated with efficacy and risk of adverse events in a real-life MS population treated with fingolimod and dimethyl fumarate. ECTRIMS Online Library. Bruschi N. 09/11/19; 279032; P672
Code availability
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Joana Ramos-Lopes has nothing to disclose.
Sónia Batista has received grant support from Biogen and speakers’ bureau fees from Biogen, Novartis, Merck, Roche, and Sanofi-Genzyme.
Inês Correia has received speakers’ bureau fees from Biogen, Novartis, Merck, Roche, Teva, and Sanofi-Genzyme.
Carla Nunes, Carmo Macário, and Lívia Sousa have received speakers’ bureau fees from Biogen, Novartis, Merck, Roche, Teva, Bayer, and Sanofi-Genzyme.
Ethical approval
The study was approved by the local Ethics Committee.
Research involving human participants
None.
Informed consent
Informed consent to patients was dispensed by the local Ethics Committee given the retrospective and merely observational nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramos-Lopes, J., Batista, S., Barradas, P. et al. Clinical effectiveness of reduced fingolimod dose in relapsing remitting multiple sclerosis—a Portuguese cohort. Neurol Sci 42, 1039–1043 (2021). https://doi.org/10.1007/s10072-020-04629-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04629-6