Abstract
Introduction
Late-Onset Pompe Disease (LOPD) is characterized by progressive limb-girdle muscle weakness and respiratory dysfunction. Diaphragm is the most impaired muscle in LOPD and its dysfunction cause major respiratory symptoms. The aim of this study was to evaluate the correlation between diaphragm thickness and mobility assessed by ultrasonography and respiratory function and muscle strength tests in patients with LOPD.
Methods
17 patients with LOPD (9 female, 47 ± 15 years) and 17 age and gender-matched healthy controls underwent spirometry, muscle strength testing, and ultrasound evaluation of diaphragm excursion and thickness.
Results
The following parameters were significantly reduced in LOPD patients versus controls (all p < 0.001): forced vital capacity (FVC) in seated and supine position, maximum inspiratory and expiratory pressure (MIP and MEP), diaphragm excursion, thickness at functional residual capacity (FRC) and total lung capacity (TLC), and thickness fraction (TF). Ultrasound studies of diaphragm thickness at FRC correlated with MIP (r = 0.74; p < 0.0001) and seated FVC(r = 0.73; p < 0.05). Diaphragm thickness at TLC correlated with MIP (r = 0.85; p < 0.0001) and FVC in both seated (r = 0.77; p < 0.0001) and supine position (r = 0.68; p < 0.05). TF correlated significantly with MIP (r = 0.80; p < 0.001), FVC in both seated (r = 0.66; p < 0,005) and supine position (r = 0.61; p < 0.05). Interestingly diaphragm thickness at FRC correlated with disease duration (years) in LOPD patients (r = −0.53; p < 0,05). Ultrasound diaphragm mobility correlated with diaphragm thickness at TLC(r = 0.87; p < 0.0001), FRC (r = 0.84; p < 0.005) and TF (r = 0.73; p < 0.05). Moreover diaphragm mobility correlated with FVC in seated(r = 0.79; p < 0.005) and supine position(r = 0.74; p < 0.05) and MIP (r = 0.81; p < 0.005).
Conclusion
Diaphragm ultrasonography is a simple and reproducible technique for manage respiratory dysfunction in LOPD patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Late-Onset Pompe Disease (LOPD), also known as glycogen storage disease type II (GSDII), is an autosomal recessive inherited disorder, due to a largely reduced activity of the lysosomal acid alpha-glucosidase (GAA). The deficiency of GAA results in a failure of lysosomal glycogen degradation, leading to a progressive accumulation in several tissues and organs [1, 2]. Progressive limb-girdle weakness and respiratory dysfunction characterize LOPD patients [3].Enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (rhGAA) is available since 2006 [4, 5] and may improve or stabilize motor and respiratory function [6]; it has also been suggested that an early initiation of ERT could delay muscle derangement [7]. New therapeutic strategies such as new generation ERT or gene therapy are underway [8].Diaphragm is the most impaired respiratory muscle in LOPD and its dysfunction causes major respiratory symptoms and later in the course of the disease, the necessity of a ventilation support [9].Invasive and non-invasive methods can be applied to evaluate diaphragm function [10].The former are laborious and difficult to be used in a follow-up program whereas the latter are preferable because are repeatable although dependent on patient cooperation. Diaphragm ultrasonography (DUS) is a relatively recent, easy to perform and reproducible technique that was utilized to evaluate diaphragm dysfunction in several respiratory diseases [11]. Considering the neuromuscular disorders, few studies have utilized DUS to investigate diaphragm function [12,13,14].Since diaphragm weakness is one of the main LOPD features, DUS could become an easy way to investigate diaphragmatic dysfunction in these patients. We perform a cross-sectional case-control study to evaluate DUS in 17 LOPD compared to healthy control volunteers, trying to match the results with traditional lung function tests actually used in routine clinical practice.
Material and methods
Study participants
The study was conducted from January 2019 to July 2019. It was approved by the Research Ethics Committee of the University of Messina School of Medicine Hospital Policlinico “G. Martino” (protocol n° 118). All patients and controls provided written informed consent prior to enrolment in the program. This study was a part of a wider project investigating diaphragmatic function by ultrasonography in neuromuscular patients. All recruited patients were adults and had a proven LOPD diagnosis according to the current recommendations [15]. All patients harbored the common mutation c.-32-13 T>G (IVS1-13 T>G), associated with a second pathogenic mutation detected in the second allele (Table 1). Control subjects were matched for age, gender, and body mass index. For assessment of neurological disability, the Walton Medwin-Gardner scale (WMG) was applied [16].The WGMS score ranges from 0 to 10, with a higher score indicating an greater disability. The modified Medical Research Council dyspnea scale (mMRC-DS) was used for self-assessment of breathlessness [17].It stratifies severity of dyspnea in respiratory diseases ranges from 0 to 4, with a higher score indicating more breathless.
Spirometry and tests of respiratory muscle strength
Forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were measured from flow-volume curves obtained with a pneumotachograph (Ganshorn Medizin Electronic GmBh, Germany) according to ATS/ERS standards in both upright and supine positions [18]. Values were expressed as absolute values and percentage of predicted normal values. Reference values were derived from published data [19]. A drop in the percentage of predicted FVC upon changing posture from the upright to the supine position > 20% was considered as expression of diaphragmatic weakness [20, 21]. Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) tests were, respectively, performed by an experienced operator with MicroRPM (Micro Medical, Kent, UK), who strongly advised the subjects to produce maximum inspiratory (Mueller maneuver) and expiratory (Valsalva maneuvers) efforts at near residual volume and total lung capacity, respectively. All tests were repeated at least three times and highest value measured was recorded. Reference values were taken into account according to previously published data [22].
Diaphragm ultrasound
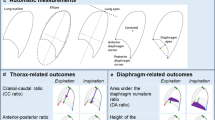
Subjects were examined in the semi-recumbent position with the bed slope at 45°. A high resolution portable ultrasound machine (MicroMaxx; Sonosite Inc., Bothell, WA, USA) was used, with a 7–13 MHz linear array transducer. Real time ultrasound was used to identify the zone of apposition where the diaphragm was most easily visualized. Typically, the eighth or ninth intercostal space was chosen, between the antero-axillary and mid-axillary lines [23]. The diaphragm was identified by its characteristic 3-layered appearance, location beneath the intercostal muscles and subcutaneous tissue, and extension deep to the ribs (as opposed to the intercostal muscles which span the ribs). The normal diaphragm is composed of a relatively thick layer of hypoechoic (dark) muscle tissue encased between 2 hyperechoic (bright) lines of pleural and peritoneal fascia. The hypoechoic muscle will thicken significantly during deep inspiration (Fig. 1a) compared to thickness at end expiration (Fig. 1b). At more cephalad intercostal spaces, the diaphragm will become obscured by the very hyperechoic shadow of the lung coming into the field of view with deep inspiration (curtain sign). The thickness of diaphragm was measured near the zone of identification of curtain sign. Once the most appropriate intercostal space was identified, the subject was instructed to breathe quietly, while 3 images were captured at functional residual capacity (FRC). The subject was then instructed to take slow deep breaths in and out, and 3 images were captured at total lung capacity (TLC). Electronic calipers were used to measure the thickness of the diaphragm muscle at the aspect closest to the more caudal rib, where the 2 fascial lines outlining the diaphragm were parallel, and the 3 images for each position were then averaged to give a thickness at FRC (ThFRC) and a thickness at TLC (ThTLC). We also calculated the thickening fraction (TF), as the proportional thickening of the diaphragm from FRC to TLC. TF represents an index of diaphragmatic thickening as is defined by the following equation: TF = ([ThTLC − ThFRC]/ThFRC) × 100. To evaluate diaphragm mobility echocardiograph was connect to a 2.5 to 3.5 MHz transducer array and M-mode was selected. Right hemidiaphragm was chosen for best liver acoustic view [24]. The probe was placed between the midclavicular and anterior axillary lines, in the subcostal area, and directed medially, cranially, and dorsally, so that the ultrasound beam reached perpendicularly the posterior third of the right hemidiaphragm (Fig. 2). Diaphragm movements were recorded in M-mode. Ultrasonographic measurements were performed during quiet breathing (QB) and deep breathing (DB). This maneuver began at the end of normal expiration, and the volunteers were asked to breathe in as deeply as they possibly could. At least 3 measurements for each parameter were taken. Again, the averaged value of three consecutive measurements was recorded for each. Images were stored in electronic format by an examiner blinded to the analysis and to the results of lung tests.
Statistical analysis
All analyses and graphical illustrations were performed using GraphPad Prism 7™ (GraphPad Software, San Diego, California). Results are expressed as mean and standard deviation for parametric data. Categorical variables are expressed as percentages, unless otherwise specified. Differences between groups were analyzed using the unpaired t -test or the Mann-Whitney U test as appropriate. The Pearson correlation coefficient was used to evaluate correlations between different parameters. For all analyses, a p value of < 0.05 was considered statistically significant.
Results
Subjects
17 patients with LOPD (age 47 ± 15 years, 9 female, BMI 27.0 ± 6 kg/m2) and 17 healthy control subjects (age 47 ± 14 years, 8 male, BMI 26 ± 5 kg/m2) were enrolled (Table 2). Each patient underwent DUS examination as well as pulmonary function tests (PFTs) in the same day. Mean disease duration was 12 ± 8 years and age at disease diagnosis was 34 ± 14 years. When the clinical evaluation was performed, 13/17 patients presented a limb-girdle muscle weakness with a prominent involvement of axial and proximal muscles at lower limbs. Four of 17 had only increased serum CK and myalgia. Respiratory involvement was more evident in 10/17 patients with history of exercise intolerance and exertional dyspnea (mean MMRC 2.5, interquartile range 2–3). Eight of 10 patients required noninvasive ventilation (NIV) (mean usage time: 8 h/night) for nocturnal hypoventilation. Thirteen of 17 patients were receiving ERT according to standard recommendations (biweekly, 20 mg/kg body weight). Duration of ERT ranged from 1 year to 10 years.
Spirometry and tests of respiratory muscle strength
The results of spirometry and respiratory strength tests in LOPD patients and healthy controls are summarized in Table 2. The following parameters were significantly reduced in LOPD patients versus controls: FEV1 (both expressed as absolute value and % of predicted) in seated and supine position, FVC (both expressed as absolute value and % of predicted) in seated and supine position. Mean drop in FVC passing from seated to supine position was significantly different in LOPD patients compared to healthy controls [− 0,56 ± 0.41 L (27 ± 19% pred) vs 0,120 ± 0.06 L (3 ± 1% pred), p < 0.0001]. LOPD patient n. 1,3,6 did not perform supine spirometry due to increased dyspnea and inability to maintain the supine position. Evaluation of muscle strength revealed a significant reduction of MIP [45 ± 24 cmH2O (52 ± 23%pred) vs 98 ± 16 cmH2O(112 ± 10%pred), p < 0.0001] and MEP [60 ± 31 cmH2O (60 ± 24%pred) vs 134 ± 16 cmH2O(109 ± 28%pred), p < 0.0001]comparing LOPD patients and healthy control. LOPD patient n. 6 did not perform MIP and MEP maneuvers for incapacity to sealing the mouthpiece.
Diaphragm ultrasonography
Whereas diaphragm thickness has been measured in all subjects except one LOPD patient for lack of cooperation, quantitative assessment of diaphragm excursion was hampered by an insufficient acoustic windows in a subset of 5 LOPD patients. Diaphragm mobility during deep breathing, ThTLC, ThFRC, and TF were significantly reduced in LOPD patients versus healthy controls (p < 0.0001) (Table 3 and Fig. 3). As an example ThFRC, ThTLC, and diaphragm mobility images in a LOPD patient are reported in Fig. 4.
Correlation between diaphragm ultrasonography, functional scales, and disease duration
Statistically significant correlations were found between mMRC-DS and diaphragm mobility (r = − 0.64; p < 0.05), ThFRC (r = −0.20; p < 0.05), MIP (r = − 0.58; p < 0.05), FVC (r = − 0.53; p < 0.05), FVCsup (r = − 0.71; p < 0.05), and FVC drop from sitting to supine position (r = − 0.75; p < 0.05). The WGMS was correlated significantly with MIP% pred (r = − 0.68; p < 0.05), FVC%pred (r = −0.55; p < 0.05) and FVC drop from sitting to supine position (r = 0.59; p < 0.05). Finally disease duration expressed in years was correlated with ThFRC (r = − 0.53; p < 0.05).
Correlations between diaphragm ultrasonography, spirometry, and respiratory muscle strength tests
Statistically significant correlations were found between ThTLC with MIP (r = 0.85; p < 0.0001), FVC both in seated (r = 0.77; p < 0.0001) and supine position (r = 0.68; p < 0.05). ThFRC correlated significantly with MIP (r = 0.74; p < 0.0001) and FVC (r = 0.73; p < 0.05). TF correlated significantly with MIP(r = 0.80; p < 0.001), FVC in seated (r = 0.66; p = 0,005), and supine position (r = 0.61; p = 0.034).Ultrasound diaphragm mobility correlated with diaphragm thickness at TLC(r = 0.87; p < 0.0001), FRC (r = 0.84; p < 0.005), and TF (r = 0.73; p < 0.05). Moreover diaphragm mobility correlated with FVC in seated (r = 0.79; p < 0.005) and supine position(r = 0.74; p < 0.05) and MIP (r = 0.81; p < 0.005) Significant correlations between diaphragm ultrasonography, spirometry, and respiratory muscle strength tests in LOPD patient are reported in figs. 5 and 6.
Role of NIV
Of the 17 LOPD, 8 patients used NIV (mean usage time: 8 h/night) for nocturnal hypoventilation. Statistical analysis of patients divided by the use of NIV or not showed that patients on NIV had a significant reduction (p < 0.05) of ultrasound diaphragm mobility, ThTLC (p < 0.05), ThFRC, and TF. Moreover LOPD patients using NIV had a significant reduction (p < 0.05) of FVC in seated and supine position and its variation in percentage compared with LOPD patients with no NIV. Finally LOPD on NIV patients had a significant reduction (p < 0.05) of inspiratory muscle strength evaluated by MIP, but no statistically significant variation was found for MEP. Differences between diaphragm ultrasonography, spirometry, and respiratory muscle strength tests in LOPD patients with and without NIV are reported in Fig. 7.
Discussion
This cross-sectional case-control study comprehensively explored, in patients with LOPD, diaphragm function evaluated by ultrasonoghraphy. The results showed that ultrasonography assessment of diaphragm function is a rapid and easy technique to perform even at bedside with a significant correlation with the traditional respiratory tests used in daily clinical practice. Several authors focused attention on a potential role of DUS in the evaluation of diaphragm activity. Determination of diaphragm thickness and real-time diaphragm excursions by ultrasonography is an easy and reproducible method and need minimum patient cooperation [11, 25]. Considering its use in neuromuscular disorders, ultrasonography has already been utilized to evaluate diaphragm muscle weakness in patients with amyotrophic lateral sclerosis (ALS) [12], Duchenne muscular dystrophy (DMD) [14], and other muscle disorders [26]. Recently, in a case of Facio-Scapulo-Humeral Distrophy (FSHD) there was an evidence of a significant reduction of diaphragm excursion amplitude and thickening ratio compared with an healthy control group [27]. Unfortunately, routine PFTs, including FVC reduction between upright and supine position as well as MIP and MEP reflects an indirect way to assess diaphragm muscle dysfunction in LOPD. Whereas the findings obtained from a small subgroup of 4 presymptomatic patients (2 females) in absence of respiratory symptoms suggest that diaphragm weakness could evaluated by DUS as an early and initial sign. Therefore, this technique can be applied in routine clinical practice for evaluation of diaphragm function at the early stage of the disease. However, diaphragmatic CT and MRI are reference imaging tests to explore diaphragm integrity. We have previously demonstrated by CT and MRI studies that diaphragm involvement represents the predominant cause of respiratory failure in LOPD usually with preservation of other respiratory muscles [28]. However these procedures have some limitations: CT exposes patients to radiations whereas for MRI evaluation, although is quite fast and not invasive, is difficult to be performed in patients with severe respiratory insufficiency that cannot stand long supine. On the other hand ultrasonography can be used at bedside [29]. Recently Spiesshoefer and coworkers [30] investigated diaphragm morphology and function by ultrasonography in 13 LOPD patients. They primarily investigated the neural contributions to diaphragm dysfunction but evidence of phrenic nerve involvement was not found, but the study was not designed to correlate diaphragm function evaluated by ultrasonography with routine PFTs. In the present study we confirm that thickness and mobility of diaphragm are significantly reduced in LOPD patients compared with healthy controls resulting in a good clinical correlation with the traditional PFTs. 4 LOPD patients included in this study were not receiving ERT because still presymptomatic and presenting only with hyperCKemia. This small number of patients without ERT were not comparable with the symptomatic patients, but when related to the healthy control group they showed a reduction in thickness fraction (110 ± 22 vs 181 ± 49, respectively, p < 0,05) and diaphragm mobility (6.5 ± 1 mm vs 7.8 ± 0.4, respectively, p < 0,05). Consequently, our data confirm that diaphragm ultrasonography is an alternative useful and risk-free technique to supply clinical, functional, and neurophysiological assessment of respiratory muscle weakness in LOPD patients. Despite the comprehensive protocol applied in this study, some limitations have to be recognized. Firstly, intra-observer variability of diaphragm ultrasonography could affect the results. However we minimized this bias by an extensive operator training and by performing up to three reproducible tests per patient. Secondly, we did not perform a comparison with other methods that may be considered a gold standard in the assessment of diaphragmatic function as trandiaphragmatic pressures because, in our opinion, those are invasive procedures and quite difficult to be performed in a routine clinical practice. However a previous study found a good correlation between transdiaphragmatic pressure time product and diaphragm thickness [31].
In conclusion, we suggested that DUS should become a routine method to evaluate diaphragm dysfunction in LOPD patients. Interestingly, DUS could help physicians in anticipating diagnosis of subclinical dysfunction of the diaphragm in LOPD patients. If these data are confirmed in further clinical trials, DUS could be part of the diagnostic pathway in LOPD patients for the management of respiratory failure, including indication for noninvasive ventilation as well as a useful outcome measure for future diagnostic and therapeutic trials.
References
Montagnese F, Barca E, Musumeci O, Mondello S, Migliorato A, Ciranni A, Rodolico C, De Filippi P, Danesino C, Toscano A (2015) Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): unusual features and response to treatment. J Neurol 262(4):968–978. https://doi.org/10.1007/s00415-015-7664-0
Toscano A, Rodolico C, Musumeci O (2019) Multisystem late onset Pompe disease (LOPD): an update on clinical aspects. Annals of translational medicine 7(13):284. https://doi.org/10.21037/atm.2019.07.24
van der Ploeg AT, Reuser AJ (2008) Pompe's disease. Lancet 372(9646):1342–1353. https://doi.org/10.1016/S0140-6736(08)61555-X
Toscano A, Schoser B (2013) Enzyme replacement therapy in late-onset Pompe disease: a systematic literature review. J Neurol 260(4):951–959. https://doi.org/10.1007/s00415-012-6636-x
Schoser B, Stewart A, Kanters S, Hamed A, Jansen J, Chan K, Karamouzian M, Toscano A (2017) Survival and long-term outcomes in late-onset Pompe disease following alglucosidase alfa treatment: a systematic review and meta-analysis. J Neurol 264(4):621–630. https://doi.org/10.1007/s00415-016-8219-8
van der Ploeg AT, Clemens PR, Corzo D, Escolar DM, Florence J, Groeneveld GJ, Herson S, Kishnani PS, Laforet P, Lake SL, Lange DJ, Leshner RT, Mayhew JE, Morgan C, Nozaki K, Park DJ, Pestronk A, Rosenbloom B, Skrinar A, van Capelle CI, van der Beek NA, Wasserstein M, Zivkovic SA (2010) A randomized study of alglucosidase alfa in late-onset Pompe's disease. N Engl J Med 362(15):1396–1406. https://doi.org/10.1056/NEJMoa0909859
Angelini C, Semplicini C, Ravaglia S, Bembi B, Servidei S, Pegoraro E, Moggio M, Filosto M, Sette E, Crescimanno G, Tonin P, Parini R, Morandi L, Marrosu G, Greco G, Musumeci O, Di Iorio G, Siciliano G, Donati MA, Carubbi F, Ermani M, Mongini T, Toscano A, Italian GG (2012) Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J Neurol 259(5):952–958. https://doi.org/10.1007/s00415-011-6293-5
Ronzitti G, Collaud F, Laforet P, Mingozzi F (2019) Progress and challenges of gene therapy for Pompe disease. Ann Transl Med 7(13):287. https://doi.org/10.21037/atm.2019.04.67
Gaeta M, Barca E, Ruggeri P, Minutoli F, Rodolico C, Mazziotti S, Milardi D, Musumeci O, Toscano A (2013) Late-onset Pompe disease (LOPD): correlations between respiratory muscles CT and MRI features and pulmonary function. Mol Genet Metab 110(3):290–296. https://doi.org/10.1016/j.ymgme.2013.06.023
Mustfa N, Moxham J (2001) Respiratory muscle assessment in motor neurone disease. QJM 94(9):497–502. https://doi.org/10.1093/qjmed/94.9.497
Sarwal A, Walker FO, Cartwright MS (2013) Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve 47(3):319–329. https://doi.org/10.1002/mus.23671
Pinto S, Alves P, Pimentel B, Swash M, de Carvalho M (2016) Ultrasound for assessment of diaphragm in ALS. Clin Neurophysiol 127(1):892–897. https://doi.org/10.1016/j.clinph.2015.03.024
Fantini R, Mandrioli J, Zona S, Antenora F, Iattoni A, Monelli M, Fini N, Tonelli R, Clini E, Marchioni A (2016) Ultrasound assessment of diaphragmatic function in patients with amyotrophic lateral sclerosis. Respirology 21(5):932–938. https://doi.org/10.1111/resp.12759
De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB (1997) Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax 52(5):472–475. https://doi.org/10.1136/thx.52.5.472
van der Ploeg AT, Kruijshaar ME, Toscano A, Laforet P, Angelini C, Lachmann RH, Pascual Pascual SI, Roberts M, Rosler K, Stulnig T, van Doorn PA, Van den Bergh PYK, Vissing J, Schoser B, European Pompe C (2017) European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: a 10-year experience. Eur J Neurol 24(6):768–e731. https://doi.org/10.1111/ene.13285
Angelini C, Semplicini C, Ravaglia S, Moggio M, Comi GP, Musumeci O, Pegoraro E, Tonin P, Filosto M, Servidei S, Morandi L, Crescimanno G, Marrosu G, Siciliano G, Mongini T, Toscano A, Italian Group on G (2012) New motor outcome function measures in evaluation of late-onset Pompe disease before and after enzyme replacement therapy. Muscle Nerve 45(6):831–834. https://doi.org/10.1002/mus.23340
Horowitz MB, Mahler DA (1998) Dyspnea ratings for prescription of cross-modal exercise in patients with COPD. Chest 113(1):60–64. https://doi.org/10.1378/chest.113.1.60
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET (2005) Standardisation of spirometry. Eur Respir J 26(2):319–338. https://doi.org/10.1183/09031936.05.00034805
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, Initiative ERSGLF (2012) Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 40(6):1324–1343. https://doi.org/10.1183/09031936.00080312
Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dube BP, Fauroux B, Gea J, Guenette JA, Hudson AL, Kabitz HJ, Laghi F, Langer D, Luo YM, Neder JA, O'Donnell D, Polkey MI, Rabinovich RA, Rossi A, Series F, Similowski T, Spengler CM, Vogiatzis I, Verges S (2019) ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J 53(6). https://doi.org/10.1183/13993003.01214-2018
Fromageot C, Lofaso F, Annane D, Falaize L, Lejaille M, Clair B, Gajdos P, Raphael JC (2001) Supine fall in lung volumes in the assessment of diaphragmatic weakness in neuromuscular disorders. Arch Phys Med Rehabil 82(1):123–128. https://doi.org/10.1053/apmr.2001.18053
Wilson SH, Cooke NT, Edwards RH, Spiro SG (1984) Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax 39(7):535–538. https://doi.org/10.1136/thx.39.7.535
Boon AJ, Harper CJ, Ghahfarokhi LS, Strommen JA, Watson JC, Sorenson EJ (2013) Two-dimensional ultrasound imaging of the diaphragm: quantitative values in normal subjects. Muscle Nerve 47(6):884–889. https://doi.org/10.1002/mus.23702
Testa A, Soldati G, Giannuzzi R, Berardi S, Portale G, Gentiloni Silveri N (2011) Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol 37(1):44–52. https://doi.org/10.1016/j.ultrasmedbio.2010.10.004
Fayssoil A, Behin A, Ogna A, Mompoint D, Amthor H, Clair B, Laforet P, Mansart A, Prigent H, Orlikowski D, Stojkovic T, Vinit S, Carlier R, Eymard B, Lofaso F, Annane D (2018) Diaphragm: pathophysiology and ultrasound imaging in neuromuscular disorders. Journal of neuromuscular diseases 5(1):1–10. https://doi.org/10.3233/JND-170276
Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T (2016) Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve 53(4):522–527. https://doi.org/10.1002/mus.24902
Henke C, Spiesshoefer J, Kabitz HJ, Herkenrath S, Randerath W, Brix T, Gorlich D, Young P, Boentert M (2019) Respiratory muscle weakness in facioscapulohumeral muscular dystrophy. Muscle Nerve 60(6):679–686. https://doi.org/10.1002/mus.26717
Gaeta M, Musumeci O, Mondello S, Ruggeri P, Montagnese F, Cucinotta M, Vinci S, Milardi D, Toscano A (2015) Clinical and pathophysiological clues of respiratory dysfunction in late-onset Pompe disease: new insights from a comparative study by MRI and respiratory function assessment. Neuromuscular disorders : NMD 25(11):852–858. https://doi.org/10.1016/j.nmd.2015.09.003
Harlaar L, Ciet P, van der Ploeg AT, Brusse E, van der Beek N, Wielopolski PA, de Bruijne M, Tiddens H, van Doorn PA (2018) Imaging of respiratory muscles in neuromuscular disease: a review. Neuromuscul Disord 28(3):246–256. https://doi.org/10.1016/j.nmd.2017.11.010
Spiesshoefer J, Henke C, Kabitz HJ, Brix T, Gorlich D, Herkenrath S, Randerath W, Young P, Boentert M (2019) The nature of respiratory muscle weakness in patients with late-onset Pompe disease. Neuromuscular disorders : NMD 29(8):618–627. https://doi.org/10.1016/j.nmd.2019.06.011
Vivier E, Mekontso Dessap A, Dimassi S, Vargas F, Lyazidi A, Thille AW, Brochard L (2012) Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med 38(5):796–803. https://doi.org/10.1007/s00134-012-2547-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approved by the local Research Ethics Committee.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Ruggeri, P., Lo Monaco, L., Musumeci, O. et al. Ultrasound assessment of diaphragm function in patients with late-onset Pompe disease. Neurol Sci 41, 2175–2184 (2020). https://doi.org/10.1007/s10072-020-04316-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04316-6