Abstract
Background
Based on the results of randomized, double-blind, placebo-controlled trials, the benefit and safety of edaravone in the treatment of amyotrophic lateral sclerosis remain controversial. We performed a meta-analysis to evaluate the efficacy and safety of edaravone in the treatment of this disease.
Methods
We searched PubMed, the Cochrane Library, and Embase from the inception of electronic data to April 2018. We included randomized, double-blind, placebo-controlled trials reporting amyotrophic lateral sclerosis patients receiving 60-mg intravenous edaravone or intravenous saline placebo for 24 weeks. The primary efficacy evaluation was changed in Amyotrophic Lateral Sclerosis Functional Rating Scale score from baseline to after the trial. Measure of safety was the frequency of investigated adverse events and serious adverse events. Data synthesis and analysis and evaluation of risk of bias were performed using RevMan 5.3 software. Heterogeneity among studies was evaluated with the I2 statistic.
Results
A total of 367 patients were analyzed across three randomized controlled trials (183 patients receiving intravenous edaravone; 184 receiving placebo). A difference in ALSFRS-R score between groups at 24 weeks was found (mean difference [MD] = 1.63, 95% confidence interval [CI] 0.26–3.00, P = .02). No differences in the frequency of adverse events (odds ratio [OR] = 1.22, 95% CI 0.68–2.19, P = .50) or serious adverse events (OR = 0.71, 95% CI 0.43–1.19, P = .20) were found.
Conclusion
Intravenous edaravone is efficacious in amyotrophic lateral sclerosis patients, with no severe adverse effects. Additional reliable randomized controlled trials with larger sample sizes will further assess the efficacy and safety of edaravone in amyotrophic lateral sclerosis.
Clinical trial registration
The systematic review and meta-analysis was registered in the international prospective register of systematic reviews. (PROSPERO registration number: CRD42018096191; http://www.crd.york.ac.uk/PROSPERO.)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease that is refractory and progresses rapidly [1, 2]. It is characterized by progressive loss of motor neurons in the anterior horn of the spinal cord [3]. Muscular atrophy and reduced muscle strength are two primary symptoms. Approximately 90–95% of reported ALS cases are sporadic, whereas 5–10% of all cases are familial [4]. Median survival times are reported as 20–48 months from the onset of symptoms [5]. The etiology of ALS is unknown, and thus, the identification of causal genes and environmental factors remains elusive.
ALS brings considerable distress to patients and families, as well as economic burden not only to those affected by the disease but also to health systems. However, there are few treatments available. Riluzole, an anti-glutamatergic agent, is the mainly used available therapeutic treatment for ALS, improving life expectancy by a mere 3–6 months [6, 7]. At present, the progress of ALS is largely dependent on symptomatic treatment and multidisciplinary care. Because of the limited choice of ALS treatment drugs, research into effective drugs is intense. Increasing evidence indicates that oxidative stress induced by free radicals plays a key role in the development and progression of ALS [8, 9]. The central nervous system is vulnerable to free radicals, owing to its high oxygen consumption, low free radical-scavenging ability, and poor regenerative capacity [10, 11]. Reactive oxygen species (ROS) initiate the apoptotic process by influencing intracellular calcium ion content and injuring specific cell molecules, releasing excitatory and toxic amino acid cross-linking metabolites. In addition, ROS affects the synthesis of DNA, causing gene mutations by altering the activities of specific enzymes such as glutamate synthetase and superoxide dismutase [12, 13].
Edaravone (MCI-186,3-methyl-1-phenyl-2-pyrazolin-5-one; Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan) is a free radical-scavenging drug that has been proved to eliminate lipid peroxides and hydroxyl radicals that damage endothelial and neuronal cells [14, 15]. The molecular weight of this lipophilic drug is low, so, blood–brain barrier permeability is up to 60% [16]. Edaravone suppresses the death of motor neurons and glial cells by stimulating the production of prostacyclin and the inflammatory media production of leukotrienes, restraining lipid peroxidation and reducing the concentration of free radicals. Its lipid peroxidation restraining properties have been proved in vitro [17]. Studies have confirmed the favorable effects of edaravone in wobbler mice with ALS-like symptoms and in animal models of ALS, with amelioration of the decline of motor function and delays to the progression of symptoms [15, 18]. The antioxidant effects of edaravone in ALS patients have also been investigated in several clinical trials. In an open-label investigator-initiated study, Yoshino and Kimura [19] found that the level of 3NT in cerebrospinal fluid was significantly lower after 14 days of treatment with edaravone than at baseline. In addition, Nagase et al. [20] found that peroxynitrite may be scavenged in ALS patients after receiving edaravone. Therefore, edaravone appears to be a promising candidate to slow the progression of ALS in patients.
Some published clinical data from ALS patients have demonstrated efficacy for edaravone, but other data have not. Based on this background, we performed a meta-analysis of data from all available randomized controlled trials (RCTs) of edaravone in ALS patients, to help determine its safety and efficacy in ALS treatment [21,22,23].
Materials and methods
This systematic review was performed according to the Systematic Reviews and Meta-Analyses (PRISMA) statement in File 1.
Data sources and search strategy
The first author conducted a systematic search of PubMed, Embase, and the Cochrane Library from the inception of electronic data to April 2018, for all relevant clinical trials published in the English language. Ongoing or unpublished studies were excluded. The search terms and strategies are shown in File 2.
Study selection and data extraction
Included studies needed to satisfy the following criteria: (1) The study design was randomized, double-blind, parallel-group, and placebo-controlled; (2) The age range of patients was 20–75 years; (3) Included patients had a diagnosis of “definite,” “probable,” “probable laboratory-supported,” or “possible” ALS, according to the revised Airlie House diagnostic criteria, or grades 1 to 3 according to the Japan ALS severity classification; (4) Included patients had a forced vital capacity (FVC%) of at least 60%; and (5) During the pre-observation period (12 weeks) prior to study drug administration, the change in revised ALS Functional Rating Scale (ALSFRS-R) score was between − 1 and − 4 points. A study was excluded if unpublished or lacking a control group. Two investigators conducted the data extraction independently. Any discrepancies were discussed with a third investigator until a consensus was reached.
A standardized data extraction form was used to extract the eligible study characteristics and the quality and outcome data. Data characteristics included country (centers), study period, edaravone doses, treatment time, sample size, patient age, sex, weight, diagnosis (E1 Escorial World Federation of Neurology criteria), initial symptoms, duration of disease, ALSFRS-R score, and riluzole use (Table 1).
Risk of bias
Two reviewers independently performed the study quality analysis, and disagreement was resolved through discussion between the two reviewers or by consultation with a third reviewer. We assessed the quality of the included studies using the risk of bias assessment tools developed by the Cochrane Collaboration, covering the following six domains: sequence generation, allocation concealment, blinding, incomplete data, selective reporting, and other bias. The quality of each study was categorized into the following three degrees of risk: low, unclear, or high [24].
Statistical analysis
Data analysis was carried out using RevMan 5.3 software (Nordic Cochrane Centre and the Cochrane Collaboration, Copenhagen, Denmark). We used I2 index to assess for between-study heterogeneity. We selected a random effect model to perform the meta-analysis if I2 > 50%, considered to be significant heterogeneity. Otherwise, a fixed effect model would be selected. In addition, we had the option to perform a sensibility analysis to explore the origins of heterogeneity if necessary. The effect of edaravone on outcome was expressed as mean difference (MD) and weighted mean difference with 95% confidence intervals (CIs). For safety outcome, risk difference (odds ratio, OR) with 95% CI was applied. The presence of publication bias was determined by funnel plots.
Results
Literature search
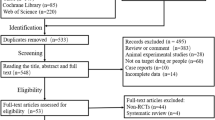
The initial literature search found 79 articles, from which duplicates were removed using EndNote software (version 7.5; Niles Software). By screening the titles and abstracts of the remaining 65 studies carefully, 59 articles were excluded. The excluded articles included reports of animal and cell experiments, articles that did not report on edaravone research, reviews and commentaries, and reports of trials with a lack of clear outcome. After scanning the articles, three were removed for not being true RCTs. Finally, three studies [21,22,23] were retained involving a total of 183 cases in the edaravone group and 184 cases in the placebo group. The flow diagram shown in File 3 indicates the study selection process.
Description of studies
The included studies [21,22,23] were all randomized, double-blind, placebo-controlled trials performed by the same teams from Japan. Each RCT compared efficacy and safety between edaravone and placebo among ALS patients. Clinical trial design and the dosage of edaravone among groups were the same across the studies. Two studies selected patients with grades 1 or 2 ALS according to the Japan ALS severity classification, while the other study included patients with grade 3 ALS. The sample size of these three studies ranged from 25 to 205 patients. According to our analysis, 183 received intravenous edaravone and 184 received placebo. Additionally, 328 patients already on riluzole continued to receive riluzole if the regimen remained unchanged. The remaining 39 patients who did not receive riluzole before the trial were prohibited from using it during the trial.
Study quality evaluation
In accordance with the Cochrane Handbook, low, high or unclear grades were given for quality in seven fields: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias [25]. In the absence of any information about risk of bias, we described the grade as unclear. Two reviewers independently conducted the quality evaluation and resolved any disagreement by discussion with a third reviewer. As there were less than ten studies, we did not produce funnel plots to assess publication bias (Figs. 1 and 2).
Outcome results
The included studies [21,22,23] were performed by the same team and the clinical trials were continuous, so patients’ characteristics were similar. The median age of patients was 59 years, and females made up 39.0% of the study population. The average duration of disease ranged from 1.06 to 2.25 years. The baseline ALSFRS-R score before the observation period was between 36 and 44, and between 32 and 42 at the end of 12 weeks’ observation. A total of 328 included patients continued to receive riluzole according to the original protocol. The treatment time and the dosage of edaravone was the same across the three studies. (Table 1).
Efficacy
Primary endpoint
Analysis of the change in ALSFRS-R scores from baseline to the end of the sixth cycle was the primary endpoint. The three included RCTs reported least-squares MD in mean ALSFRS-R scores and heterogeneity among the studies was low (I2 = 11%, P = .32). Therefore, we chose a fixed effects model for the analysis. The results revealed a statistically significant difference between patients receiving edaravone and placebo (MD = 1.63, 95% CI 0.26–3.00, P = .02) (Fig. 3).
Secondary endpoints
Secondary endpoints in the study were ALSAQ-40 score, FVC%, Modified Norris Scale (limb or bulbar), grip strength, and pinch strength. There was no significant difference in ALSAQ-40 score between the edaravone and placebo groups (MD = − 4.74, 95% CI − 11.18–1.70, P = .15), and no significant heterogeneity was found (I2 = 0%, P = .44). There was no significant difference in FVC% between the two groups (MD = 2.99, 95% CI − 1.46–7.44, P = .19), and no significant heterogeneity was noted (I2 = 0%, P = .56). There was no significant difference in Modified Norris Scale score between the two groups (MD = 2.99, 95% CI − 0.72–6.69, P = .11), and no significant heterogeneity was detected (I2 = 0%, P = .62). The results showed no significant increase in grip strength and pinch strength between the two groups (MD = 0.44, 95% CI − 0.69–1.57, P = .45; MD = 0.09, 95% CI − 0.17–0.35, P = .49), and no significant heterogeneity was found for these two outcome measures (I2 = 0%, P = .81; I2 = 0%, P = .57) (Figs. 4, 5, 6, 7, and 8).
Safety
Adverse events
Each study reported on differences in adverse events (AEs) between treatment and placebo groups. No significant heterogeneity was found (I2 = 0%, P = .43) and a fixed effects model was used. AEs were not more frequently found in patients treated with edaravone versus placebo (OR = 1.22; 95% CI 0.68–2.19; P = .43) (Fig. 9).
Serious adverse events
The pooled results did not indicate a significant difference between groups in the number of serious adverse events (SAEs) (OR = 0.71, 95% CI 0.43–1.19, P = .02). A fixed effects model was adopted because no significant heterogeneity was detected among the studies (P = .72, I2 = 0%) (Fig. 10).
Discussion
The ALSFRS was specifically designed for the clinical evaluation of ALS patients, with a tested reliability [26]. It has been used in clinical examinations as well as evaluation of the efficacy of clinical trials [26,27,28]. In our meta-analysis, we used the change in ALSFRS-R score as a primary endpoint for evaluating the efficacy of edaravone in ALS patients, and all included studies reported this measure. The ALSFRS-R is the revised version of the ALSFRS that incorporates the items for evaluating respiratory function. The ALSFRS-R scores of patients with ALS, which is a progressive disease, are known to decrease almost linearly over the course of disease [29]. The most exciting finding of the meta-analysis was that the change in ALSFRS-R score from baseline to the end of the sixth treatment cycle was significantly different between edaravone and placebo groups.
We found no significant differences between groups for any of the secondary endpoints. The included RCTs (Abe 2014 and Abe 2017 [grade 3]) [22, 23] failed to demonstrate any statistically significant effect in any of the secondary endpoints. The other study (Abe 2017) [21] successfully demonstrated a difference for total Modified Norris Scale and ALSAQ-40 scores. It should be noted that Abe 2017 [21] focused on grades 1 or 2 patients with a confirmed or probable diagnosis of ALS and those with probable laboratory-supported ALS were not included. So, success in secondary endpoint difference in Abe 2017 [21] might have been associated with the strict inclusion criteria chosen. Overall, the results suggest that edaravone may delay the progression of functional disorders in ALS patients at early stages (grades 1 to 3).
Numbers of AEs was similar in the two groups (edaravone group: 156/184; placebo group: 152/184). All AEs were mild to moderate and considered by investigators as unrelated to the study drug. In the edaravone and placebo groups, the proportions of patients who developed SAEs during treatment were 17.4% (32/184) and 22.8% (42/184), respectively. There was no imbalance in the overall incidence of treatment-emergent SAEs between the two groups. In both groups, the most frequently seen treatment-emergent SAEs were dysphagia, respiratory disorder, speech disorder, and pneumonia aspiration. The SAEs were not considered by the investigators to be associated with edaravone, and all were attributed to deteriorating ALS. Therefore, we can conclude that edaravone has a favorable safety profile.
During the first six cycles (24 weeks) of the three studies, we found that edaravone slowed the progression rate of ALSFRS-R, a global functional measure of disability, and identified no safety problems to which we should pay careful attention. However, there are several limitations to our meta-analysis. First, it is not established whether long-term edaravone therapy prolongs survival. Second, the studies included patients who were previously given riluzole and did not consider pharmacological interactions between edaravone and riluzole during the RCTs [5]. Third, this meta-analysis included 342 patients with grades 1 or 2 ALS (Japan ALS severity classification) and 25 patients with a grade 3 classification; therefore, we were unable to assess the efficacy of edaravone in advanced ALS patients. Oxidative stress may play a role in ALS from the early stages to the advanced stage [30, 31]. Whether edaravone is safe and effective in more patients with advanced ALS requires further studies. Finally, the included patients were all Japanese; we look forward to more RCT results from other geographical populations.
The included studies in our systematic review were double-blind, parallel-group, randomized trials. This suggests that the results of this systematic review are reliable and accurate. However, the search terms and strategies were in English, which restricted the meta-analysis to English language trials. This raises the risk of publication bias.
Conclusion
Our research suggests that 24 weeks’ treatment with edaravone in ALS patients (grades 1–3 Japan ALS scale) has a favorable safety and encouraging efficacy profile, when compared with placebo. However, more well-designed RCTs with a larger sample size are needed to explore the long-term efficacy of edaravone and potential AEs.
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
Amyotrophic lateral sclerosis functional rating scale
- RevMan:
-
Review manager
- RCTs:
-
Randomized controlled trials
- MD:
-
Mean difference
- CI:
-
Confidence interval
- OR:
-
Odds ratio
References
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955
Barber SC, Mead RJ, Shaw PJ (2006) Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta 1762(11–12):1051–1067
Taylor JP, Brown RJ, Cleveland DW, Decoding ALS (2016) From genes to mechanism. Nature 539(7628):197–206
Katyal N, Govindarajan R (2017) Shortcomings in the current amyotrophic lateral sclerosis trials and potential solutions for improvement. Front Neurol 8:521
Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG (2009) Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 10(5–6):310–323
Petrov D, Mansfield C, Moussy A, Hermine O (2017) ALS Clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front Aging Neurosci 9:68
Miller RG, Mitchell JD, Moore DH (2012) Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev (3):CD1447
Watanabe K, Tanaka M, Yuki S, Hirai M, Yamamoto Y (2018) How is edaravone effective against acute ischemic stroke and amyotrophic lateral sclerosis? J Clin Biochem Nutr 62(1):20–38
Barnham KJ, Masters CL, Bush AI (2004) Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 3(3):205–214
Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L (2017) Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxidative Med Cell Longev 2017:2525967
Duan W, Li X, Shi J, Guo Y, Li Z, Li C (2010) Mutant TAR DNA-binding protein-43 induces oxidative injury in motor neuron-like cell. Neuroscience 169(4):1621–1629
Zuo L, Zhou T, Pannell BK, Ziegler AC, Best TM (2015) Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol (Oxford) 214(3):329–348
Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, Mahata J, Kilty M, Bednarz K, Bell D, Gordon PH, Hornig M, Mehrazin M, Naini A, Flint BM, Factor-Litvak P (2008) Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler 9(3):177–183
Geffard M, Mangas A, Bedat D, Covenas R (2018) GEMALS : A promising therapy for amyotrophic lateral sclerosis. Exp Ther Med 15(4):3203–3210
Ito H, Wate R, Zhang J, Ohnishi S, Kaneko S, Ito H, Nakano S, Kusaka H (2008) Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp Neurol 213(2):448–455
Tanaka M (2002) Pharmacological and clinical profile of the free radical scavenger edaravone as a neuroprotective agent. Nihon Yakurigaku Zasshi 119(5):301–308
Fujisawa A, Yamamoto Y (2016) Edaravone, a potent free radical scavenger, reacts with peroxynitrite to produce predominantly 4-NO-edaravone. Redox Rep 21(3):98–103
Aoki M, Warita H, Mizuno H, Suzuki N, Yuki S, Itoyama Y (2011) Feasibility study for functional test battery of SOD transgenic rat (H46R) and evaluation of edaravone, a free radical scavenger. Brain Res 1382:321–325
Yoshino H, Kimura A (2009) Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study). Amyotroph Lateral Scler 7(4):247–251
Nagase M, Yamamoto Y, Miyazaki Y, Yoshino H (2016) Increased oxidative stress in patients with amyotrophic lateral sclerosis and the effect of edaravone administration. Redox Rep 21(3):104–112
Abe K, Aoki M, Tsuji S, et al. (2017) Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trials. Lancet Neurol 16(7):505–512
Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, Hamada C, Kondo K, Yoneoka T, Akimoto M, Yoshino H (2014) Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener 15(7–8):610–617
Abe K, Itoyama Y, Tsuji S, et al. (2017) Exploratory double-blind, Parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener 18(sup1):40–48
Lundh A, Gotzsche PC (2008) Recommendations by Cochrane review groups for assessment of the risk of bias in studies. BMC Med Res Methodol 8:22
Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 51.0. Available at: http://handbook.cochrane.org/.2011
(1996) The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. The ALS CNTF treatment study (ACTS) phase I-II study group. Arch Neurol 53(2):141–147
Kasarskis E, Shefner J, Miller R (1999) A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF study group (phase III). Neurology 52(7):1427–1433
Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, Sumino S, Yanagisawa N (2001) Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS (ALSFRS-R) Japanese version. No To Shinkei 53(4):346–355
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci 169(1–2):13–21
Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH (2004) Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology 62(10):1758–1765
D’Amico E, Factor-Litvak P, Santella RM, Mitsumoto H (2013) Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med 65:509–527
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, L., Song, Z., Li, X. et al. Efficacy and safety of edaravone in treatment of amyotrophic lateral sclerosis—a systematic review and meta-analysis. Neurol Sci 40, 235–241 (2019). https://doi.org/10.1007/s10072-018-3653-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3653-2