Abstract
The primary endpoint of this work was to evaluate the effect of safinamide on non-motor symptoms (NMS) in patients affected by idiopathic Parkinson’s disease (PD) complicated by motor fluctuations. We retrospectively collected data from 20 subjects affected by idiopathic PD in treatment with l-dopa alone or in combination with dopamine agonists, who began to be treated with safinamide due to the occurrence of motor fluctuations. Secondary endpoints included SCales for Outcomes in Parkinson’s disease (SCOPA) Motor Scale, cognitive assessment, the Hoehn and Yahr stage, Clinical Impression of Severity Index for Parkinson’s Disease, Hospital Anxiety And Depression Scale, Physical and Mental Fatigue, Parkinson’s disease Sleep Scale, Parkinson’s Disease Questionnaire-8 (PDQ-8) and EQ-5D. Each one of these scales/questionnaires was performed at baseline and T1. For efficacy analyses, continuous variables were treated with descriptive statistics, using mean and standard deviations. A non-parametric test (the Friedman test) was carried out to evaluate the statistical significance of the results observed. We found a statistically significant reduction of the total score of NMS, of 6 domains out of 9, and 13 items out of 30. A statistically significant reduction of SCOPA Motor Scale, PDQ-8, and CISI was also detected. In conclusion, our data showed a positive effect of safinamide on NMS and confirm its positive effect on motor symptomatology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common chronic progressive neurodegenerative disorder in the elderly after Alzheimer’s disease [1]. It results from degeneration of the substantia nigra pars compacta and the consequent dysfunction of the dopaminergic nigrostriatal pathway, but serotonergic, noradrenergic, and glutamate pathways are also affected [2, 3].
Diagnosis of PD is still mainly clinical, based on the presence of bradykinesia in combination with muscular rigidity, resting tremor, and/or postural instability [4]. Beside the cardinal motor symptoms, we find the so called non-motor symptoms (NMS), cognitive, neuropsychiatric, sleep, autonomic, and sensory disturbances, which gained attention in the last few years due to their impact on quality of life of patients and caregivers [5, 6]. The non-motor symptoms (NMS) continue to be poorly recognized and inadequately treated; they may be intrinsic to the disease pathology or may be consequences of treatment with dopaminergic agents [6]. They can be present at disease onset and some of them, such as hyposmia and psychiatric and sleep disorders, may even precede motor symptoms. The pathophysiology of NMS is still not completely characterized: it is hypothesized that a dysfunction of both dopaminergic and non-dopaminergic systems contributes to their development [5].
Levodopa (l-dopa) is so far considered the most effective treatment for the motor symptoms of PD, but its long-term use is associated with motor fluctuations and l-dopa-induced dyskinesia [7, 8]. Moreover, as the disease progresses, non-dopaminergic pathways become involved [9] and patients require add-on therapy to improve motor fluctuations without exacerbating dyskinesia.
Safinamide is an oral, once-a-day therapy, approved by the EU for the treatment of mid- to late-stage fluctuating PD as add-on therapy to a stable dose of l-dopa alone or in combination with other PD drugs. Safinamide (S)-(+)-2-[4-(3-fluorobenzyloxy-benzylamino)propionamide] is a benzylamino derivative with multiple mechanisms of action and antiparkinsonian and anticonvulsant properties. It provides a highly selective and reversible inhibition of MAO-B, and it blocks voltage-dependent sodium channels and modulates calcium channels, with inhibition of dopamine reuptake and modulation of glutamate release, thus involving both dopaminergic and glutaminergic systems [10,11,12,13,14,15,16,17]. In parkinsonian patients, safinamide has been demonstrated to significantly improve ON time with no or non-troublesome dyskinesia when used as an adjunct to l-dopa in patients with PD and motor fluctuations, for 2 years [18, 19]. Recently, Italian authors presented the first observational retrospective study reporting the effects of safinamide in the real-life setting, confirming that safinamide is an effective and safe add-on treatment for motor fluctuations and/or disabling dyskinesia. Moreover, these authors suggested safinamide as a levodopa-DA-COMT-I-sparing strategy [20]. Interestingly, several authors have also suggested a possible effect of safinamide in non-motor symptoms, as pain and mood, in particular at the dosage of 100 mg/day [21,22,23]. Treatment with safinamide should be started at 50 mg/day. This daily dose may be increased to 100 mg/day on the basis of individual clinical need [24].
The aim of this work was to evaluate the effect of safinamide on non-motor symptoms in patients affected by idiopathic Parkinson’s disease complicated by motor fluctuations.
Materials and methods
Study design and characteristics of the patients
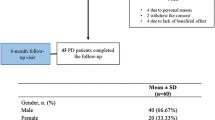
We retrospectively collected data from a small but well-characterized cohort of patients (20 subjects) affected by idiopathic Parkinson’s disease complicated by motor fluctuations, in treatment with l-dopa alone or in combination with dopamine agonist, followed up at the Movement Disorder Center of Varese, and screened between May and September 2015 (baseline T0) for the presence of non-motor symptoms in a period of stable management of the disease (no changes in therapy required). Due to the occurrence of motor fluctuations, such patients began to be treated with safinamide (50 mg/day in the first 15 days, then 100 mg/day) between March and July 2016. Then, the data were collected again at least after 3 months from the introduction of safinamide (4.4 ± 1.05 months) and in a period of stable management of the disease (no other therapeutic changes required) (T1).
Study endpoints
The primary endpoint of the study was the evaluation of a possible effect of safinamide 100 mg/day in Non-Motor Symptom Scale (NMSS) scores from baseline to T1. The NMSS assessment is obtained through patient interview and contains 30 questions (grouped into 9 domains) that are scored with respect to severity and frequency [25]. The Italian version of the Non-Motor Symptom Scale (NMSS) for Parkinson’s disease was used [26].
Secondary endpoints included motor functions, activities of daily living and motor fluctuations, assessed using SCales for Outcomes in Parkinson’s disease (SCOPA) motor scale; cognitive status was evaluated by the Mini Mental State Examination and Cognitive assessment [27]. Other endpoints were the Hoehn and Yahr stage, Clinical Impression of Severity Index for Parkinson’s Disease (CISI-PD), Hospital Anxiety and Depression Scale (HADS), Physical and Mental Fatigue (PHYS-F and MENT-F), Parkinson’s disease Sleep Scale (PDSS), Parkinson’s Disease Questionnaire-8 (PDQ-8), and EQ-5D.
Each one of these scales/questionnaires was performed at baseline (T0) and T1.
Statistical analysis
For efficacy analyses, continuous variables were treated with descriptive statistics, using mean and standard deviations.
A non-parametric Friedman test was carried out, and p values < 0.05 were considered to be statistically significant.
Results
Baseline patients’ characteristics are reported in Table 1.
Twenty patients with idiopathic PD, 10 women, mean age 75 ± 6.3 years, with the mean disease duration of 14.5 ± 6.8 years, were prescribed safinamide 50 mg/day for 15 days, then safinamide 100 mg/day, as add-on therapy to l-dopa for the occurrence of motor fluctuations, and evaluated after about 4.4 months of treatment. Seven patients were receiving rasagiline at T0: in these cases, they first withdrew rasagiline and then started safinamide 3 weeks later, in the same way of the rest of the cohort. Results of questionnaires and scales administered are reported in Table 2.
We evaluated the variation of NMS scores in terms of total score, scores of each domain (Fig. 1), and scores of the single items. The total score of NMS showed a statistically significant reduction (p = 0.00031). Analyzing the single domains, we found a statistically significant reduction of domains 1 (cardiovascular, p = 0.020), 2 (sleep/fatigue, p = 0.001), 3 (mood/cognition, p = 0.003), 5 (attention/memory, p = 0.008), 7 (urinary, p = 0.046), and 8 (sexual function, p = 0.008).
Evaluating the single NMS items, we observed a general decrease in the vast majority of the items. The reduction is up to 80% compared to the original value. A notable exception is the score for NMS11, which shows a + 89% increase at T1 and is identified as an outlier. This result is highly influenced by the strong change of scores of patient 1 that go from 1 at T0 to 12 at T1, while the rest of the patients showed almost similar scores for this item at T0 and T1. We therefore removed patient 1 from the cohort due to the outliers in its results.
We obtained a statistically significant reduction for the items 1, 3, 4, 5, 7, 8, 10, 12, 16, 22, 24, 25, and 26.
The general decrease of the single items amounts to 26%. Increases in scores can be observed for NMS13 (hallucinations) and NMS19 (sialorrhea), in the range 9–20%; however, these increases did not prove to be statistically significant.
Beside NMS, a statistically significant reduction was observed for SCOPA Motor Scale, PDQ-8, and CISI. In particular, regarding the SCOPA assessment, a general reduction in the range of – 25–− 75% was observed. The items that showed the most significant improvement were items describing postural instability, swallowing, and presence of OFF periods. Significant improvements were observed also in items describing rapid alternate movements of hands, rigidity, speech, freezing during “on,” dressing, hygiene, changing position, dyskinesia (both presence and severity), and motor fluctuations.
No adverse events occurred in our population: no one of our patient withdrew from safinamide at T1.
Discussion
Safinamide represents an important option for patients with PD already treated with l-dopa alone, or in combination with other PD treatments. Its dopaminergic and non-dopaminergic properties are a novelty among the antiparkinsonian drugs. The studies so far performed demonstrated a good safety profile and an improvement of the ON time with no or non-troublesome dyskinesia.
Our data confirm the positive effect of safinamide 100 mg/day on motor symptomatology as demonstrated by the reduction of scores in SCOP Motor Scale. We have to highlight how amelioration obtained in fluctuations, bradykinesia, and rigidity does not induce occurrence or worsening of dyskinesia.
PDQ-8 showed a significant statically reduction: in particular, item 8 addressing painful muscle cramps or spasm reduced its values. This result could be in part related to the improvement in motor and mood conditions; anyway, we can also hypothesize a direct role of safinamide on pain control due in particular to its non-dopaminergic effect [22]. This finding is particularly important because chronic pain in PD patients is often underestimated despite the high impact on quality of life of patients and caregivers.
We found a statistically significant improvement of the NMS scores, in particular for the scores regarding cardiovascular, sleep/fatigue, mood/cognition, attention/memory, and urinary and sexual function.
Sleep disorders are very frequent and heterogeneous in PD patients; sometimes, they can appear years before the occurrence of motor symptoms, and they have been related to the nigrostriatal dopaminergic degeneration [28]. A recent study by Liguori et al. described improvement of sleep disorders in parkinsonian patients treated by safinamide in contrast to those treated by rasagiline: on the basis of new experimental data and of the clinical data obtained in their study, the authors conclude that the positive effect on sleep disorders may be related to the glutamatergic pathway of safinamide [29]. Thus, we may hypothesize that safinamide can improve sleep disorders because of its dual effect; nevertheless, analyzing items 2, 14, and 15 of the PDSS, such results are not confirmed. This discrepancy can be explained by the different methodology of the questionnaires used and, of course, from the small number of patients enrolled.
Mood condition ameliorates in our patients after the introduction of safinamide: these findings are supported by the reduction of the HADS scores, even if not statistically significant. The improvement of mood may be related to the amelioration of motor symptoms but, as suggested by previous works, we should consider a possible direct effect of safinamide on mood disorders by both dopaminergic and glutamatergic mechanism [23].
Our patients referred a greater concentration after the introduction of safinamide: this finding is really interesting because may represent a starting point to explore a possible effect of safinamide on attention mechanism or in general on cognitive functions of PD patients. Actually, our data are not sufficient to address this topic but further studies with specific cognitive evaluations of patients treated by safinamide may give interesting results.
Our data showed an improvement of sexual function and urinary function: so far, no data regarding these topics are available in the literature.
We observed a slight trend toward the increase in the NMS items describing hallucinations and sialorrhea (items 13 and 19): this could represent a side effect of safinamide; in particular, the occurrence of hallucinations could be related to the increased dopaminergic stimulation. Our study take into consideration only two observations, T0 and T1, without therapeutic changes occurring in between: as suggested by other authors, safinamide can help sparing LD and DA, so this side effect could remit after the reduction of LEDD. However, especially considering that the average age was 75.0 years and the average disease’s duration was 14.5 years, we can hypothesize that the occurrence of sialorrhea and hallucinations may be related to the natural progression of the disease. Due to the small size of the cohort and the short follow-up, these data are ambiguous.
All the questionnaires and scales were performed at T1 when all patients were treated by safinamide 100 mg, so we can conclude that safinamide is effective on non-motor symptoms at this dosage; we cannot rule out a similar effect also with lower dosage but this hypothesis should be confirmed by further studies. Due to the short follow-up period, we can consider our findings as the result of the acute effect of safinamide on non-motor symptoms.
We have to acknowledge the limitations of this study: first of all, this is a retrospective study and we analyzed data from a very small cohort of patients, even if well characterized. Moreover, the observation was limited to a small period. For these reasons, our observations are obviously not conclusive and need to be confirmed at follow-up and in larger cohorts of patients. However, our results strengthen literature data [20, 22, 23] and offer new starting points.
Conclusion
Dopaminergic treatments do not help or even cause or worse non-motor symptoms. Different studies have showed positive effect of safinamide in the treatment of some disorders such as sleep [29, 30], mood, and pain [22, 23]. Based on our data and the recent literature, we suggest a significant role of safinamide in the treatment for non-motor symptoms. Considering the heterogeneity of such symptoms and their impact on the quality of life of either patients and caregivers, safinamide may be considered a valid therapeutic option even in early stages of fluctuations, as already highlighted by Mancini et al. [20]
Change history
06 December 2018
The original version of this article contains an error in Table 2. The Authors realized that they submitted the previous version of Table 2. The correct version of Table 2 is shown here.
References
Yadav HP, Li Y (2015) The development of treatment for Parkinson’s disease. Adv Parkinsons Dis 4:59–78
Schapira AH (2011) Monoamine oxidase B inhibitors for the treatment of Parkinson’s disease: a review of symptomatic and potential disease-modifying effects. CNS Drugs 25:1061–1071
Gardoni F, Bellone C (2015) Modulation of the glutamatergic transmission by Dopamine: a focus on Parkinson, Huntington and addiction diseases. Front Cell Neurosci 9:25
Grosset DG, Macphee GJ, Nairn M, Guideline Development Group (2010) Diagnosis and pharmacological management of Parkinson’s disease: summary of SIGN guidelines. DG BMJ 340:b5614
Antonini A (2009) Nonmotor symptoms in Parkinson’s disease. Eur Neurol Rev 4(2):25–27
Park A, Stacy M (2009) Non-motor symptoms in Parkinson’s disease. J Neurol 256(Suppl 3):S293–S298
Shoulson I, Glaubiger GA, Chase TN (1975) On-off response. Clinical and biochemical correlations during oral and intravenous levodopa administration in parkinsonian patients. Neurology 25:1144
Hauser RA (2009) Levodopa: past, present. and future Eur Neurol 62:1–8
Blandini F, Armentero MT (2012) New pharmacological avenues for the treatment of L-dopa-induced dyskinesias in Parkinson’s disease: targeting glutamate and adenosine receptors. Expert Opin Investig Drugs 21:153–168
Caccia C, Maj R, Calabresi M, Maestroni S, Faravelli L, Curatolo L et al (2006) Safinamide: from molecular targets to a new anti-Parkinson drug. Neurology 67(7 Suppl 2):S18–S23
Onofrj M, Bonanni L, Thomas A (2008) An expert opinion on safinamide in Parkinson’s disease. Expert Opin Investig Drugs 17:1115–1125
Binda C, Milczek EM, Bonivento D, Wang J, Mattevi A, Edmondson DE (2011) Lights and shadows on monoamine oxidase inhibition in neuroprotective pharmacological therapies. Curr Top Med Chem 11:2788–2796 Review
Fariello RG (2007) Safinamide. Neurotherapeutics 4:110–116 Review
Schapira AH (2010) Safinamide in the treatment of Parkinson’s disease. Expert Opin Pharmacother 11:2261–2268
Singer C (2012) Managing the patient with newly diagnosed Parkinson disease. Cleve Clin J Med 79(Suppl 2):S3–S7
Gregoire L, Jourdain VA, Townsend M, Roach A, Di Paolo T (2013) Safinamide reduces dyskinesias and prolongs L-dopa antiparkinsonian effect in parkinsonian monkeys. Parkinsonism Relat Disord 19:508–514
Deeks ED (2015) Safinamide: first global approval. Drugs 75:705–711
Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt M, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, Anand R, Study 016 Investigators (2014) Randomized trial of safinamide add-on to levodopa in Parkinson’s disease with motor fluctuations. Mov Disord 29:229–237
Borgohain R, Szasz J, Stanzione P, Meshram C, Bhatt MH, Chirilineau D, Stocchi F, Lucini V, Giuliani R, Forrest E, Rice P, Anand R, the Study 018 Investigators (2014) Two-year, randomized, controlled study of safinamide as add-on to levodopa in mid to late Parkinson’s disease. Mov Disord 29:1273–1280
Mancini F, Di Fonzo A, Lazzeri G, Borellini L, Silani V, Lacerenza M, Comi C (2018) Real life evaluation of safinamide effectiveness in Parkinson’s disease. Neurol Sci 39:733–739
Bonuccelli U (2015) Effects of safinamide on motor complications and pain in advancing Parkinson’s disease – post hoc analyses of pivotal trials. European Neurological Review 10(2):176–181
Cattaneo C, Barone P, Bonizzoni E, Sardina M (2017) Effects of safinamide on pain in fluctuating Parkinson’s disease patients: a post-hoc analysis. Journal of Parkinson’s Disease 7:95–101
Cattaneo C, Müller T, Bonizzoni E, Lazzeri G, Kottakis I, Keywood C (2017) Long-term effects of safinamide on mood fluctuations in Parkinson’s disease. Journal of Parkinson’s Disease 7(2017):629–634
XADAGO® (safinamide) Summary of Product Characteristics
Chaudhuri KR, Martinez-Martin P (2008) Quantitation of non-motor symptoms in Parkinson’s disease. Eur J Neurol 15:2–7
Cova I, Di Battista ME, Vanacore N, Papi CP, Alampi G, Rubino A, Valente M, Meco G, Contri P, Di Pucchio A, Lacorte E, Priori A, Mariani C, Pomati S (2017) Validation of the Italian version of the nonmotor symptoms scale for Parkinson’s disease. Parkinsonism Relat Disord 34:38–42
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Prell T (2018) Structural and functional brain patterns of non-motor syndromes in Parkinson’s disease. Front Neurol 12(9):138
Liguori C, Stefani A, Ruffini R, Mercuri NB, Pierantozzi M (2018) Safinamide effect on sleep disturbances and daytime sleepiness in motor fluctuating Parkinson’s disease patients: a validated questionnaires-controlled study. Parkinsonism Relat Disord. doi: https://doi.org/10.1016/j.parkreldis.2018.06.033
Liguori C, Mercuri NB, Stefani A, Pierantozzi M (2018) Effective treatment of restless legs syndrome by safinamide in Parkinson’s disease patients. Sleep Med 41:113–114
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bianchi, M.L.E., Riboldazzi, G., Mauri, M. et al. Efficacy of safinamide on non-motor symptoms in a cohort of patients affected by idiopathic Parkinson’s disease. Neurol Sci 40, 275–279 (2019). https://doi.org/10.1007/s10072-018-3628-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3628-3