Abstract
Abnormalities in auditory P300 test have been observed in patients with Parkinson’s disease (PD). We aimed to investigate whether or not additional electrophysiological tests assist in making the clinical diagnosis of mild cognitive impairment in Parkinson’s disease (PD-MCI), and we evaluated P300 changes in patients with non-demented PD and analyzed the correlation between the cognitive features and P300 changes. Twenty patients with PD who had been diagnosed with mild cognitive impairment (PD-MCI group) according to the Movement Disorder Society (MDS) 2012 PD-MCI level II criteria, 21 patients with PD without cognitive impairment (PD-Normal group), and 20 control subjects (control group) who were neurologically normal were examined by the standard auditory oddball paradigm. The N100, P200, N200, and P300 latencies and N100-P200, P200-N200, and N200-P300 amplitudes were measured and analyzed. P300 latencies recorded from Fz, Cz, and Pz and N200 latency recorded from Fz were significantly longer in the PD-MCI group than in the PD-Normal and the control group (respectively p < 0.001, p = 0.041). P300 amplitude recorded from Fz was significantly lower in PD-MCI group than those in the other groups (p = 0.038). While P300 was obtained in all patients in the PD-Normal and the control group, it was lost in 35% of PD-MCI patients. The results show that P300 provides a diagnostic tool for detecting PDMCI. We suggest that P300 prolongation and loss of P300 potential could be used as supportive parameter in the diagnosis of PD-MCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a common, chronic neurodegenerative disorder characterized by a combination of motor problems, as well as non-motor features such as cognitive, autonomic, and affective abnormalities [1]. Cognitive impairment is particularly prevalent in PD and varies from mild deficits to severe dementia [2]. The term “mild cognitive impairment (MCI)”, used to define the transition between the cognitive impairment observed during normal aging and dementia, is very important in predicting the development of dementia in general population and in patients with PD [3].
The Movement Disorders Society (MDS) Task Force review reported that a mean of 27% (range 19 to 38%) of non-demented patients with PD had MCI [4]. A recent study reported that 91% of the mild cognitive impairment in Parkinson’s disease (PD-MCI) cohort had progressed to Parkinson’s disease dementia (PDD) at 16 years [5]. However, diagnosing PD-MCI in clinical practice may be problematic. The MDS Task Force has published diagnostic criteria for PD-MCI in 2012 [6]. PD-MCI is a syndrome characterized by clinical, cognitive, and functional criteria based on clinical evaluation, anamnesis, and neuropsychological tests. We think that these criteria could be supported by a laboratory test in order to provide more objective results.
The event-related evoked potentials (EREPs) as a laboratory method have been used as markers for cognitive functions in patients with neurological and psychiatric disorders. Auditory P300 test provides information about the pathways related to attention and memory. P300 amplitude reflects the CNS activity during the comparison process of the given stimulus and the information of the record of that stimulus in the memory, and P300 latency reflects the rate of classification of the stimulus [7]. It has been determined that P300 amplitude decreases and the P300 peak latency increases as the severity of cognitive dysfunction increases [8, 9].
The aim of this study was to investigate P300 changes of PD-MCI patients. With the obtained results, it was aimed to contribute to the creation of laboratory-aided diagnostic approaches that would contribute to the diagnosis of MCI in PD.
Patients and method
This study included patients who presented to Akdeniz University Medical Faculty Neurology Department between 2014 and 2015 and diagnosed with PD-MCI and PD with no cognitive impairment, as well as healthy volunteers without cognitive impairment matched in terms of age, gender, and education. PD and PD-MCI were diagnosed on the basis of UK Parkinson Disease Society Brain Bank and MDS 2012 PD-MCI level II criteria, respectively. Patients’ Hoehn-Yahr (HY) stages were stages 1–3. All subjects had completed at least primary school. Exclusion criteria were as follows: PDD or other dementia syndromes, other parkinsonism-plus syndromes, heredodegenerative parkinsonism, and secondary parkinsonism, severe or unstable depression, medications affecting cognition (anticholinergics, antidepressants, or anxiolytics), other causes of cognitive impairment (e.g., seizures, strokes, head trauma), or auditory loss preventing cooperation to P300 test.
Collection of data at screening included demographic and clinical features (disease duration, current medications), the unified Parkinson’s disease rating scale (UPDRS), HY staging, Beck Depression Inventory (BDI), and standardized mini-mental state examination (MMSE).
The subjects were administered a neuropsychological test battery comprising ten tests grouped into five cognitive domains based on the MDS recommendations level II diagnostic criteria [6, 10,11,12,13,14,15,16,17,18,19]: memory (Wechsler Memory Scale Logical Memory Subtest, Öktem Verbal Memory Processes Test), attention and working memory (digit span test, Trail Making Test A-B), visuospatial function (Benton’s Judgment of Line Orientation Test, Benton Facial Recognition Test), executive function (verbal fluency test, clock drawing test), and language (Boston Naming Test, Gülhane Aphasia Test-2 Naming Subtest). In accordance with the MDS PD-MCI diagnostic criteria, patients who failed in at least two tests were included in the PD-MCI group. The patients who succeeded at all tests or failed at only one test formed the PD-Normal cognition group.
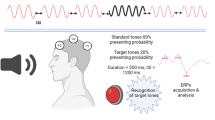
Auditory P300 recordings were made while the subjects were sitting in a partially soundproof room with their eyes open. The signals were recorded at Fz, Cz, and Pz electrode sites (10–20 International System) using Ag/AgCl electrodes fixed to the scalp, referred to linked earlobes with a forehead ground. The inter-electrode resistance was always below 5 kQ. First, the binaural audiometric thresholds were determined at 1000 Hz for each subject. The auditory stimuli consisted of 1000 Hz pure tone bursts as standard stimuli and 2000 Hz pure tone bursts as target stimuli. The tones were presented in a random series, with a stimulus interval ranging between 1.5 and 2 s. According to the “oddball” paradigm, 20% of the tones were high and designated as target stimuli. The subjects were instructed to count silently the target tones, reporting the total at the end of the session. Two blocks, containing 30 random target stimuli, were recorded in each subject. The latency and the amplitude of the P300 components were calculated in each subject as the mean of the values of the two blocks. The P300 component was defined as the most positive peak occurring within a window of 300–500 ms. A Nihon Kohden Neuropack M1 device was used to record EREPs. The N100, P200, N200, and P300 latencies and peak-to-peak N100-P200, P200-N200, and N200-P300 amplitudes were measured from Fz, Pz, and Cz electrode sites and analyzed.
All subjects gave informed consent. The study was approved by Akdeniz University and Institutional Review Board and Ethics Committee with a project code of 2014.04.0103.009.
Statistical analysis
Fisher’s exact test and Pearson’s chi-square analysis were performed for categorical variables. In order to compare quantitative variables with normal distribution, Student’s t test and ANOVA were performed. Mann-Whitney U test and Kruskal-Wallis rank sum tests were used for comparison of quantitative variables with non-normal distribution. In ANOVA, differences between the groups compared using Tukey’s honestly significant difference post hoc test and in Kruskal-Wallis rank sum tests, Bonferroni-Dunn post hoc correction was used. Spearman and Pearson correlation coefficients were calculated to find correlation between variables. Results are expressed as n (%), mean ± standard deviation, or median (minimum-maximum). The overall data were analyzed with the Statistical Package for the Social Sciences (SPSS) 18, and a p value < 0.05 was considered statistically significant.
Results
Demographic characteristics
This study evaluated the data of 20 healthy controls and two groups of patients, 20 subjects diagnosed PD-MCI and 21 PD subjects without cognitive impairment who were diagnosed by means of detailed history, clinical features, and detailed neurocognitive evaluation. Demographic and clinical features of the three groups have been presented in Table 1. The groups were similar regarding age, sex, educational status, PD duration, and HY stage. PD-MCI group was more severely impaired at UPDRS and UPDRS part III (motor examination) (p = 0.025, p = 0.040, respectively).
When the PD-MCI and PD-Normal groups were compared in terms of the context of the treatment, as if it included levodopa, dopamine agonist, and monoamine oxidase-B (MAO-B) inhibitors, no significant difference was detected between the groups in terms of dopamine agonist and MAO-B inhibitor use. Levodopa was found in the treatment of 12 patients (60%) in the PD-MCI group and in the treatment of 6 patients (28.6%) in the PD-Normal group, and this finding was found to be statistically significant (p = 0.043).
P300 latencies and amplitudes
The N100, P200, N200, and P300 latencies and the N100-P200, P200-N200, and N200-P300 peak-to-peak amplitude values of participants that were recorded from the Fz, Cz, and Pz electrodes were evaluated. The potentials that could not be achieved were excluded from the study, and the difference between the latency and amplitude measurements between the groups was analyzed.
In Fig. 1, the sample P300 traces of participants, one from each of the control (a), PD-Normal (b), and PD-MCI (c) groups, and the P300 trace of one participant in the PH-MCI group, whose P300 potential could not be achieved (d), can be seen.
From the records, the P300 potentials of six patients (30%) from the Fz and Cz electrodes in the PD-MCI group could not be achieved, while in the PD-Normal and control groups, the potentials were achieved from all of the participants (p = 0.007 [PD-MCI–PD-Normal], p = 0.008 [PD-MCI–control]). From the Pz electrode, the P300 potential could not be achieved in seven patients (35%) in the PD-MCI group, while in PD-Normal and control groups, it was achieved from all of the participants (p = 0.003 [PD-MCI–PD-Normal], p = 0.004 [PD-MCI–control]). In the records made from all three electrodes, the inability to achieve P300 potentials in the PD-MCI group was statistically significant.
When the latency measurements were evaluated, the N200 and P300 latencies recorded from the Fz electrodes (p = 0.041, p < 0.001, respectively), the P300 latency recorded from the Cz electrode (p < 0.001), and the P300 latency recorded from the Pz electrode (p < 0.001) were found to be statistically significantly prolonged in the PD-MCI group, compared to the PD-Normal and control groups.
The peak-to-peak N100-P200, P200-N200, and N200-P300 amplitudes measured from the traces with Fz, Cz, and Pz electrodes were evaluated. The N200-P300 amplitudes measured with Fz electrodes in the control group were found to be higher compared to the PD-MCI group, and this value was statistically significant (p = 0.038). The data of all latency and amplitude values have been presented in Table 2.
Next, the correlation of latency and amplitude values between age and clinical parameters (PD duration, HY stage, UPDRS, and UPDRS part III scores) was examined. There was a significant positive correlation between age and Cz-P300 (r = 0.291; p = 0.031), Fz-N200 (r = 0.257; p = 0.049), and Pz-N200(r = 0.275; p = 0.034) latencies. PD duration and Fz-N200 (rho = 0.330, p = 0.040), HY stage and Pz-P200-N200 amplitude (rho = 0.320, p = 0.044), and UPDRS part III score and Pz-P200-N200 amplitude (rho = 0.340, p = 0.032) were found significantly positive correlated.
Discussion
Auditory P300 test was first defined by Sutton et al. in 1965 and became the subject of many studies, since it was an indicator of neural events related to cognitive functions [20]. There are various studies in literature using the auditory P300 test to investigate the cognitive functions in PD with or without dementia, in the early or advanced stages of the disease, in the “on” and “off” periods of the patients, and prior to or after dopaminergic treatment, and they have reported the relationship between the cognitive situation in PD and changes in P300 [21,22,23,24,25,26,27,28,29,30,31,32,33,34]. However, after the publishing of the PD-MCI criteria by MDS, there is no study using auditory P300 test in PD-MCI patients diagnosed based on these criteria. The primary goal of our study was to investigate whether or not there was a change in the auditory P300 test findings observed in PD-MCI patients and in PD patients with no cognitive impairment.
In P300 test as a response to standard sound, there is one negative N100 wave with the peak between 90 and 200 ms after the initiation of stimulus and one following positive P200 wave. This complex is generated in the primary auditory cortex and represents an exogenous (“stimulus-dependent”) cortical auditory potential. This complex is followed by N200-P300 complex composed of one negative and one positive wave. In the record that is registered as a response to “targeted” stimuli, the dominant component is P300 wave. It is a positive wave whose latency in healthy persons is 300–500 ms and represents a neurophysiological parameter that is in correlation with the speed of cognitive processes. It is supposed that P300 waves are generated by the medial structure of the temporal lobus, that is, by the hippocampal formation and the temporoparietal associative zone. P300 is generated when attention is directed to the processing of a new stimulus that is different from the mental model of the expected stimuli. P300 wave latency corresponds to the speed of stimuli classification based on discrimination between two events, when there is an adaptation of the mental model of structure of the stimulus to the actual event [35, 36].
We determined that the late endogenous component P300 latencies recorded from Fz, Cz, and Pz electrodes were prolonged in the PD-MCI group compared to the remaining two groups. Another late endogenous component N200 latencies were prolonged in the PD-MCI group compared to the remaining groups as well; however, this difference was significant only in the recordings obtained from the Fz electrode. The P300 amplitudes were smaller in the PD-MCI group compared to the remaining groups, and this difference was significant only in the recordings obtained from the Fz electrode. No difference was observed between the groups with regard to the latency and amplitudes of the early exogenous components N100 and P200 waves.
In a study comparing 40 PD patients without cognitive impairment and healthy controls, the P300 latency was found to be delayed in the patient group and the P300 amplitude was found to be decreasing [22]. Similarly, in another study comparing 45 PD patients without dementia, with normal controls, the P300 latency was found to be delayed in PD patients [23]. Although the term MCI was not used in any of these two studies, it was suggested that P300 may be useful in detecting subclinical cognitive impairment. In another study comparing PD patients without dementia and with healthy controls, the P200 and N200 latencies were found to be delayed in the patient group in contrast to the commonly obtained outcomes; however, no difference was found between the groups with regard to the P300 latencies. According to these results, the investigators have suggested that the possible delay in P300 latency may be a corrected parameter with l-DOPA treatment, while the delay in P200 and N200 latencies may have not been corrected [32]. In our study, PD patients were evaluated according to the PD-MCI criteria of MDS; thus, we presented the P300 differences between the patients with MCI and those with no cognitive impairment for the first time. The most important finding of our study was the delay in P300 latency in PD-MCI patients. Furthermore, we showed that there was no P300 difference between the PD patients with no cognitive impairment and healthy controls. This result suggests that the delay in the P300 latency occurs parallel to the occurrence of MCI in PD.
There is a general consensus that P300 latency of auditory event-related potentials increases with age by 0.9–1.7/year, but no clear evidence is reported about the effect of age on the amplitude of the P300 [7, 37]. In our study, a positive correlation was found with P300 latency recorded from Cz, but not on the other recording sites, and there were no correlation between age and P300 amplitude measurements.
In our results, we did not find any correlation between disease duration, HY stage, total and motor UPDRS scores, and P300 latencies. This data suggests that prolongation of the P300 latency may occur even in early stages and years of the PD, if cognitive decline is positive. In two studies which reported positive correlation between PD stage and P300 latencies, advanced stage PD patients were also participated, but in ours, patients stage were HY stages 1–3 [38, 39]. As well as in other studies, the poor correlation between P300 latencies and clinical motor score indicated that P300 delay is due to a mechanism which is presumably different from those responsible for the motor disability [38, 40].
In a study comparing PD patients matched with healthy controls according to age and gender using the P300 test, no P300 potential could be obtained in 16.6% of the patients [33]. In our study, P300 potential was obtained in all of the patients with no cognitive impairment and healthy controls, whereas it was not obtained in 35% of the patients with PD-MCI. Therefore, we believe that unavailability of this potential alone may be an indicator of the cognitive impairment.
Another finding demonstrating that the P300 test is a sensitive indicator in detecting the cognitive changes in patients with PD is that the test yields different results in the “on” and “off” periods of the disease. In 1989, a group of patients with PD with serious motor fluctuations were evaluated with the P300 test, the reaction time, and neuropsychological tests in the “on” and “off” periods. The P300 latency demonstrated a significant shortening in the “on” period, whereas no difference was observed in the reaction time and neuropsychological tests [35]. The cognitive improvement in “on” period could be shown by shortening in the P300 latency. Since the participants in our study were in the early stage of PD and they had no motor fluctuations, no evaluation could be made with this respect.
The effect of dopaminergic treatment on cognition in PD is controversial. It is thought that possible positive or negative effects of dopaminergic treatment on cognition could be demonstrated with changes in P300. In a study on this subject, patients recently diagnosed as early stage PD without dementia were evaluated prior and after l-DOPA treatment with P300 test. Following dopaminergic treatment, the P300 latency was shortened on the 15th day, whereas it was prolonged at the 3rd month and 6th month measurements [21]. In our study, the dopaminergic treatments the participants received were compared, and in the PD-MCI group, a higher number of patients had received l-DOPA treatment. However, since our participants were evaluated once using the P300 test without no change in the treatment method, no comment could be made on the effects of dopaminergic treatment on P300.
The results obtained in our study and the data in the literature together suggest that the P300 test provides an objective evaluation of the cognitive situation in PD. In PD, it is important to monitor cognitive status even in early stages or without any complaint about cognition. Although neuropsychological tests provide very valuable data about cognitive status in PD, P300 test is simple, non-invasive, and also does not necessitate the use of motor functions that are impaired in PD. In this regard, in clinical practice, P300 latency alterations could be used with neuropsychological tests to follow up cognitive status in patients with PD.
In this study, a prolonged P300 latency and an unavailable P300 potential were determined to be sensitive parameters in distinguishing PD-MCI patients from those PD patients without cognitive impairment. With prospective follow-up studies and data, this hypothesis can be supported more strongly. In conclusion, we suggest that with further and wider studies, the changes in P300 can be helpful to the diagnosis of PD-MCI and may be used as a supportive tool.
References
Zesiewicz TA, Sullivan KL, Hauser RA (2006) Nonmotor symptoms of Parkinson’s disease. Exp Rev Neurother 6:1811–1822
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y et al (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194
Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B et al (2011) MDS task force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov Disord 26(10):1814–1824
Hobson P, Meara J (2015) Mild cognitive impairment in Parkinson's disease and its progression onto dementia: a 16-year outcome evaluation of the Denbighshire cohort. Int J Geriatr Psychiatry 30(10):1048–1055
Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC et al (2012) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356
Polich J, Herbst KL (2000) P300 as a clinical assay: rationale, evaluation and findings. Int J Psychophysiol 38(1):3–19
Polich J, Ehlers CL, Otis S, Mandell AJ, Bloom FE (1986) P300 latency reflects the degree of cognitive decline in dementing illness. Electroencephalogr Clin Neurophysiol 63:138–144
Ball SS, Marsh J, Schubart G, Brown WS, Standburg R (1989) Longitudinal P300 latency changes in Alzheimer’s disease. J Gerontol 44:195–200
Wechsler D, Stone CP (1945) Wechsler Memory Scale Manual. Psychological Corporation, New York
Öktem Ö (1992) Sözel Bellek Süreçleri Testi (SBST)-Bir Önçalışma. Nöropsikiyatri Arşivi 29:196–206
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological Assessment, 4th edn. Oxford University Press, New York
Cangoz B, Karakoc E, Selekler K (2009) Trail making test: normative data for Turkish elderly population by age, sex and education. J Neurol Sci 283(1–2):73–78
Benton AL, Varney NR, Hamsher KD (1978) Visuospatial judgment: a clinical test. Arch Neurol 35(6):364–367
Keskinkılıç C (2008) Standardization of Benton face recognition test in a Turkish normal adult population. Turk J Neurol 14(3):179–190
Golden CJ, Espe-Pfeifer P, Wachsler-Felder J (2000) Neuropsychological Interpretations of Objective Psychological Tests. Kluwer Academic/Plenum Publishers, New York
Royall DR, Mulroy AR, Chiodo LK, Polk MJ (1991) Clock drawing is sensitive to executive control: a comparison of six methods. J Gerontol B Psychol Sci 54:328–333
Kaplan E, Goodglass H, Weintraub S (1983) Boston Naming Test, 2nd edn. Lea & Febiger, Philadelphia
Maviş İ, Colay K, Topbaş S, Tanrıdağ O (2007) Standardization, validity and reliability study of Gülhane Aphasia Test-2 (GAT-2). Turk J Neurol 13(2):89–98
Sutton S, Braren M, Zubin J, John ER (1965) Evoked-potential correlates of stimulus uncertainty. Science 150(3700):1187–1188
Prabhakar S, Syal P, Srivastava T (2000) P300 in newly diagnosed non-dementing Parkinson’s disease: effect of dopaminergic drugs. Neurol India 48(3):239–242
Koberskaia NN, Zenkov LR, Iakhno NN (2003) Cognitive potential p300 in Parkinson disease. Zh Nevrol Psikhiatr Im SS Korsakova 103(8):42–49
Katsarou Z, Bostantjopoulou S, Kimiskidis V, Rossopoulos E, Kazis A (2004) Auditory event-related potentials in Parkinson’s disease in relation to cognitive ability. Percept Mot Skills 98:1441–1448
Toda K, Tachibana H, Sugita M, Konishi K (1993) P300 and reaction time in Parkinson’s disease. J Geriatr Psychiatry Neurol 6(3):131–136
Hayashi R, Hanyu N, Shindo M, Tamaru F, Yanagisawa N (1993) Event-related potentials, reaction time, and cognitive state in patients with Parkinson’s disease. Adv Neurol 60:429–433
Aotsuka A, Weate SJ, Drake ME Jr, Paulson GW (1996) Event-related potentials in Parkinson’s disease. Electromyogr Clin Neurophysiol 36(4):215–220
Hayashi R, Hanyu N, Kurashima T, Tokutake T, Yanagisawa N (1996) Relationship between cognitive impairments, event-related potentials, and motor disability scores in patients with Parkinson’s disease: 2-year follow-up study. J Neurol Sci 141(1–2):45–48
Raudino F, Garavaglia P, Beretta S, Pellegrini G (1997) Auditory event-related potentials in Parkinson’s disease. Electromyogr Clin Neurophysiol 37(7):409–413
Bodis-Wollner I, Borod JC, Cicero B, Haywood CS, Raskin S, Mylin L et al (1995) Modality dependent changes in event-related potentials correlate with specific cognitive functions in nondemented patients with Parkinson’s disease. J Neural Transm Park Dis Dement Sect 9(2–3):197–209
Fattapposta F, Cordischi MV, D'Alessio C, Foti A, Amabile G (1990) Parkinson disease and cognitive evoked potentials. Riv Neurol 60(6):240–242
Sartucci F, Guerrini V, Tognoni G, Massetani R, Murri L, Muratorio A (1990) P300 and Parkinson disease. The role of cognitive changes. Riv Neurol 60(6):229–233
Ebmeier KP, Potter DD, Cochrane RHB, Crawford JR, Stewart L, Calder SA et al (1992) Event related potentials, reaction time, and cognitive performance in idiopathic Parkinson’s disease. Biol Psychol 33(1):73–89
Starkstein SE, Esteguy M, Berthier ML, Garcia H, Leiguarda R (1989) Evoked potentials, reaction time and cognitive performance in on and off phases of Parkinson’s disease. J Neurol Neurosurg Psychiatry 52:338–340
Hansch EC, Syndulko K, Cohen SN, Goldberg ZI, Potvin AR, Tourtellotte WW (1982) Cognition in Parkinson disease: an event-related potential perspective. Ann Neurol 11:599–607
Stamenović J, Đurić S, Đorđević G, Đurić V (2005) The correlation between neuropsychological and neurophysiological parameters in early stages of Parkinson’s disease. Med Biol 12(2):104–112
Jiang S, Qu C, Wang F, Liu Y, Qiao Z, Qui X et al (2015) Using event-related potential P300 as an electrophysiological marker for differential diagnosis and to predict the progression of mild cognitive impairment: a meta-analysis. Neurol Sci 36:1105–1112
Kügler CFA, Taghavy A, Platt D (1993) The event-related P300 potential analysis of cognitive human brain aging: a review. Gerontology 39:280–303
Stanzione P, Semprini R, Pierantozzi M et al (1998) Age and stage dependency of P300 latency alterations in non-demented Parkinson’s disease patients without therapy. Electroencephalogr Clin Neurophysiol 108:80–91
Md SL, Ad SM, Nóbrega AC (2014) Delayed latencies of auditory evoked potential P300 are associated with the severity of Parkinson’s disease in older patients. Arq Neuropsiquiatr 72(4):296–300
Matsui H, Nishinaka K, Oda M, Kubori T, Udaka F (2007) Auditory event-related potentials in Parkinson’s disease: prominent correlation with attention. Parkinsonism Relat Disord 13:394–398
Funding
This study was funded by Akdeniz University Research Fund with a project code of 2014.04.0103.009.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All subjects gave informed consent. The study was approved by Akdeniz University and Institutional Review Board and Ethics Committee with a project code of 2014.04.0103.009.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yilmaz, F.T., Özkaynak, S.S. & Barçin, E. Contribution of auditory P300 test to the diagnosis of mild cognitive impairment in Parkinson’s disease. Neurol Sci 38, 2103–2109 (2017). https://doi.org/10.1007/s10072-017-3106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3106-3