Abstract
Visual and semi-quantitative assessments of 123I-FP-CIT single-photon emission computed tomography (SPECT) are useful for the diagnosis of dopaminergic neurodegenerative diseases (dNDD), including Parkinson’s disease, dementia with Lewy bodies, progressive supranuclear palsy, multiple system atrophy, and corticobasal degeneration. However, the diagnostic value of combined visual and semi-quantitative assessment in dNDD remains unclear. Among 239 consecutive patients with a newly diagnosed possible parkinsonian syndrome who underwent 123I-FP-CIT SPECT in our medical center, 114 patients with a disease duration less than 7 years were diagnosed as dNDD with the established criteria or as non-dNDD according to clinical judgment. We retrospectively examined their clinical characteristics and visual and semi-quantitative assessments of 123I-FP-CIT SPECT. The striatal binding ratio (SBR) was used as a semi-quantitative measure of 123I-FP-CIT SPECT. We calculated the sensitivity and specificity of visual assessment alone, semi-quantitative assessment alone, and combined visual and semi-quantitative assessment for the diagnosis of dNDD. SBR was correlated with visual assessment. Some dNDD patients with a normal visual assessment had an abnormal SBR, and vice versa. There was no statistically significant difference between sensitivity of the diagnosis with visual assessment alone and semi-quantitative assessment alone (91.2 vs. 86.8%, respectively, p = 0.29). Combined visual and semi-quantitative assessment demonstrated superior sensitivity (96.7%) to visual assessment (p = 0.03) or semi-quantitative assessment (p = 0.003) alone with equal specificity. Visual and semi-quantitative assessments of 123I-FP-CIT SPECT are helpful for the diagnosis of dNDD, and combined visual and semi-quantitative assessment shows superior sensitivity with equal specificity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dopaminergic neurodegenerative diseases (dNDD) include Parkinson’s disease (PD), dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), multiple system atrophy (MSA), and corticobasal degeneration (CBD). In dNDD, progressive degeneration of dopaminergic cells in the substantia nigra reduces dopamine transporter (DAT) density and causes various neurological signs, e.g., tremor, rigidity, bradykinesia, and postural instability. However, non-dNDD, such as normal pressure hydrocephalus (NPH), essential tremor (ET), drug-induced parkinsonism (DIP), and cerebrovascular parkinsonism (CVP), could also show symptoms that mimic dNDD, so the clinical discrimination of dNDD from non-dNDD is sometimes difficult. 123I-FP-CIT single-photon emission computed tomography (SPECT) visualizes DAT density, and visual and semi-quantitative assessments of 123I-FP-CIT SPECT are useful for the diagnosis of dNDD [1,2,3]; however, the diagnostic value of combined visual and semi-quantitative assessment in dNDD remains unclear. The purpose of this study was to verify the diagnostic value of combined visual and semi-quantitative assessment in the diagnosis of dNDD.
Methods
Subject population
Two hundred and thirty-nine consecutive patients with a newly diagnosed possible parkinsonian syndrome, e.g., tremor, rigidity, bradykinesia, and postural instability, were investigated with 123I-FP-CIT SPECT at Kobe City Medical Center General Hospital (KCMCGH) from June 2014 to January 2016. We excluded 26 patients with a disease duration greater than 7 years, to focus on patients with a short disease duration. Among the 213 patients with a disease duration less than 7 years, 91 patients who fulfilled the established criteria were diagnosed as dNDD (PD, DLB, PSP, MSA, or CBD), and 23 patients were clinically diagnosed as non-dNDD (NPH, ET, DIP, or CVP) by experienced neurologists. Ninety-nine patients who neither fulfilled the established criteria of dNDD nor were clinically diagnosed as non-dNDD were excluded. PD, DLB, PSP, MSA, and CBD patients fulfilled the UK PD Brain Bank criteria [4], consensus guidelines for the clinical diagnosis of probable and possible DLB [5], second consensus statement on the diagnosis of MSA [6], NINDS-Society for PSP, Inc. diagnostic criteria [7], and criteria for the diagnosis of CBD [8], respectively. We retrospectively examined their clinical characteristics (age, sex, disease duration, and disease severity) and visual and semi-quantitative assessments of 123I-FP-CIT SPECT. The striatal binding ratio (SBR) was used as a semi-quantitative measure of 123I-FP-CIT SPECT [9]. The lower side of the SBR for each patient was used for the analysis. Disease severity was assessed using the Hoehn and Yahr scale. For this type of study, formal consent was not required, and this study was performed in accordance with the guidelines of the ethics committee of KCMCGH.
Visual assessment
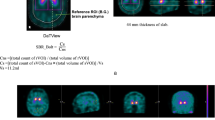
123I-FP-CIT SPECT images were visually classified by a board-certified radiologist (M.H.) blinded to the diagnosis, clinical information, and semi-quantitative assessment, according to the following scale [10] (Fig. 1): normal, symmetric uptake in the caudate and putamen; grade 1, asymmetrical loss of a putaminal tail—“comma with full stop”; grade 2, bilateral loss of putaminal tails—“two full stops”; and grade 3, partial to complete loss of the caudate and putaminal signals—“disappearing full stops.”
SPECT acquisition, data analysis, and semi-quantitative assessment
123I-FP-CIT (DatSCAN; Nihon Medi-Physics, Tokyo, Japan) of 167 MBq was intravenously administered. Images were acquired at 3 h post injection with a dual-head gamma-camera (Discovery NM/CT 670; GE Healthcare) equipped with an extended low-energy, general-purpose, collimator using a parallel head configuration [11]. The gamma camera was calibrated for a 159-keV photo peak and ±10% energy window. SPECT acquisition was performed into a 128 × 128 matrix, zoom ×1.3 (pixel size 3.4 × 3.4 mm), dynamic SPECT mode, orbit of 15-cm radius, and 32-min total acquisition time (90 projections; 10 s per projections; 4 rotations). With CT-based attenuation correction, images were reconstructed using ordered subset expectation maximization (OSEM; subset 10, iteration 6) together with a Butterworth filter cut-off frequency of 0.55 cycles/cm and scatter correction (130 keV ± 10% energy window) and re-oriented for AC-PC angle in accordance with CT images. All reconstructed transaxial slices were used for quantification by the software DaTView (AZE). DaTView uses a region of interest (ROI) approach in which the SBR is derived from a measure of total striatal counts that takes into account the partial volume effect. The definition of the image for analysis was automated and included transaxial slices within a “slab” approximately 44 mm thick centered on the highest striatal signal. The reference region was defined automatically from non-specific uptake in the whole brain enclosed in a slab, with the exclusion of the striatal region [9].
Receiver operating characteristic (ROC) analysis was performed in 114 patients who were diagnosed as dNDD or non-dNDD to define an adequate cut-off point of the SBR for the discrimination of dNDD from non-dNDD patients. ROC analysis indicated the best cut-off point of the SBR at 5.13 for the discrimination of dNDD from non-dNDD patients with a sensitivity of 86.8% and specificity 95.7% (see Supplementary Fig. 1); therefore, we classified an “SBR of no more than 5.13” as “abnormal” in this study.
Statistical analysis
The Mann–Whitney U test or chi-square test was used, as appropriate, to compare the clinical characteristics and results of 123I-FP-CIT SPECT between the dNDD and non-dNDD groups. For the comparison of disease severity, it was classified into three groups: Hoehn and Yahr scale stage 1–2, stage 3, and stage 4–5 groups. Spearman’s rank correlation was used to compare visual assessment and SBR in all 114 patients. The sensitivity and specificity for the diagnosis of dNDD with semi-quantitative assessment alone, visual assessment alone, and combined visual and semi-quantitative assessment were calculated. For diagnosis with combined visual and semi-quantitative assessment, we assessed “patients with an abnormality in either visual assessment or SBR” as “abnormal.” McNemar’s test was used to compare the sensitivity and specificity of the diagnosis with each method. P values less than 0.05 were considered statistically significant for each test. All statistical analyses were performed using JMP 10 software for Windows (SAS Institute, Cary, NC).
Results
For the 114 patients, 65 (57.0%) were male, median age was 74 years (interquartile range [IQR], 67–80 years), median disease duration was 2 years (IQR, 1–3 years), and the median Hoehn and Yahr stage was stage 3 (IQR, stage 2–3).
Clinical characteristics, visual assessment, and SBR
Table 1 shows the clinical characteristics, SBR, and visual assessment of each group. There was no significant difference among the dNDD and non-dNDD groups in sex, age, and disease severity, but disease duration was longer in the dNDD group than in the non-dNDD group (p < 0.001). The median SBR of the dNDD group was lower than that of the non-dNDD group (3.84 vs. 7.67, respectively, p < 0.001). The SBR was negatively correlated with visual assessment in all 114 patients (Table 2: p < 0.001, r = 0.701).
Diagnosis with visual and semi-quantitative assessment
As mentioned earlier, the sensitivity of the diagnosis with the SBR was 86.8% and specificity was 95.7% with the best cut-off point of 5.13. Conversely, the sensitivity of the diagnosis with visual assessment was 91.2%, and specificity was 95.7%. In the dNDD patients, 9 of the 12 patients with a normal SBR had an abnormal visual assessment, and 5 of the 8 patients with a normal visual assessment had an abnormal SBR. The sensitivity of the diagnosis with combined visual and semi-quantitative assessment was 96.7% and specificity was 95.7%. There was no statistically significant difference between the sensitivity of the diagnosis with visual assessment alone and semi-quantitative assessment alone (p = 0.29). Combined visual and semi-quantitative assessment demonstrated superior sensitivity to visual assessment alone (p = 0.03) and SBR alone (p = 0.003) with equal specificity (Fig. 2).
Discussion
In this study, the SBR of the dNDD group was lower than that of the non-dNDD group, and the SBR was negatively correlated with visual assessment. Some dNDD patients with a normal visual assessment had an abnormal SBR, and vice versa. There was no statistically significant difference in sensitivity between diagnosis with visual assessment alone and semi-quantitative assessment alone, but combined visual and semi-quantitative assessment demonstrated superior sensitivity with equal specificity.
123I-FP-CIT SPECT and post-mortem pathological studies have shown that PD patients in the early stages exhibit a decline of DAT density in the unilateral dorsal putamen, contralateral to the clinically affected side, and then in more advanced stages, this decline of DAT density proceeds to the ipsilateral putamen and caudate [12, 13]. The alterations observed in visual assessment and SBR in 123I-FP-CIT SPECT reflect such progressive degeneration of dopaminergic neurons. In the present study, the SBR of the dNDD group was significantly lower than that of the non-dNDD group, as reported previously [13], although this might be affected by the longer disease duration of the dNDD group because the SBR is correlated with disease duration [14, 15]. A correlation between visual assessment and SBR has also been reported previously [16, 17], and our results confirmed this finding.
To the best of our knowledge, the diagnostic value of combined visual and semi-quantitative assessment of 123I-FP-CIT SPECT in the diagnosis of dNDD has never been reported. Visual assessment seems to be subjective, but the interobserver agreement rates of visual assessment for “normal” or “abnormal (grades 1–3)” are sufficiently high [16, 18], which ensures the high robustness and reproducibility of visual assessment. In this study, some dNDD patients with a normal SBR have an abnormal visual assessment, which could be partly because the SBR is vulnerable to many factors such as attenuation or scatter correction methods, reconstruction, ROI drawing procedure, SPECT acquisition, and data analysis [19,20,21], but some dNDD patients with a normal visual assessment had an abnormal SBR, perhaps because of the technical limitations of visual assessment, and the combination of visual assessment and SBR demonstrated superior sensitivity with equal specificity. Therefore, we speculate that visual and semi-quantitative assessments are complementary to each other, and combined visual and semi-quantitative assessment leads to a more accurate diagnosis of dNDD.
This study has several limitations. First, the sample size of our cohorts is relatively small. The future trials in larger cohorts are necessary to validate our findings. Secondly, different diseases including PD, DLB, PSP, MSA, or CBD are mixed together in one group as dNDD, mainly due to the small sample size of our cohorts. Future studies should assess each disease separately in order to establish the utility of 123I-FP-CIT SPECT in each disease. Thirdly, because the three methods (visual, semi-quantitative, and combined assessment) were not independent, we did not conduct multiple comparison correction. However, there were still possibilities of increased alpha error. Finally, diagnostic accuracy is also potential limitation. Although we used the established criteria for PD, DLB, PSP, MSA, and CBD for the dNDD patients, the non-dNDD patients were clinically diagnosed without any established criteria. Moreover, there was no pathological confirmation of diagnosis, and a discrepancy between the clinical diagnosis and post-mortem pathological diagnoses might exist. Additionally, the diagnosis of non-dNDD might be less accurate than the diagnosis of dNDD because it might be influenced by the results of 123I-FP-CIT SPECT.
Conclusion
Visual and semi-quantitative assessments of 123I-FP-CIT SPECT are helpful for the diagnosis of dNDD, and combined visual and semi-quantitative assessment shows superior sensitivity with equal specificity. More detailed clinical analyses and accumulation of cases are necessary to elucidate the diagnostic value of combined visual and semi-quantitative assessment of 123I-FP-CIT SPECT.
References
Catafau AM, Tolosa E (2004) Impact of dopamine transporter SPECT using 123I-ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 19:1175–1182
Bajaj N, Hauser RA, Grachev ID (2013) Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 84:1288–1295
Benamer HTS, Patterson J, Grosset DG (2000) Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]FP-CIT SPET imaging: the[123I]FP-CIT SPET study group. Mov Disord 15:503–510
Hughes AJ, Daniel SE, Kilford L et al (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 65:1863–1872
Gilman S, Wenning GK, Low PA et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Litvan I, Agid Y, Calne D et al (1996) NINDS-Society for PSP, Inc. (SPSP) diagnostic criteria. Neurology 47:1–9
Armstrong MJ, Litvan I, Lang AE et al (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503
Tossici-Bolt L, Hoffmann SM, Kemp PM et al (2006) Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging 33:1491–1499
FDA prescribing information for DaTscan website. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022454sOrig1s000Lbl.pdf. Accessed 9 Jan 2017
Seret A, Nguyen D, Bernard C (2012) Quantitative capabilities of four state-of-the-art SPECT-CT cameras. EJNMMI Res 2:45
Utiumi MA, Felicio AC, Borges CR et al (2012) Dopamine transporter imaging in clinically unclear cases of parkinsonism and the importance of scans without evidence of dopaminergic deficit (SWEDDs). Arq Neuropsiquiatr 70:667–673
Piggott MA, Marshall EF, Thomas N et al (1999) Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain 122:1449–1468
Benamer HTS, Patterson J, Wyper D et al (2000) Correlation of Parkinson’s disease severity and duration with [123I]FP-CIT SPECT striatal uptake. Mov Disord 15:692–698
Pirker W (2003) Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord 18:43–51
Davidsson A, Georgiopoulos C, Dizdar N et al (2014) Comparison between visual assessment of dopaminergic degeneration pattern and semi-quantitative ratio calculations in patients with Parkinson’s disease and atypical parkinsonian syndromes using DaTSCAN® SPECT. Ann Nucl Med 28:851–859
Ottaviani S, Tinazzi M, Pasquin I et al (2006) Comparative analysis of visual and semi-quantitative assessment of striatal [123I]FP-CITSPET binding in Parkinson’s disease. Neurol Sci 27:397–401
Papathanasiou N, Rondogianni P, Chroni P et al (2012) Interobserver variability, and visual and quantitative parameters of (123)I-FP-CIT SPECT (DaTSCAN) studies. Ann Nucl Med 26:234–240
Dickson JC, Tossici-Bolt L, Sera T et al (2010) The impact of reconstruction method on the quantification of DaTSCAN images. Eur J Nucl Med Mol Imaging 37:23–35
Staff RT, Ahearn TS, Wilson K et al (2009) Shape analysis of 123I-N-omega-fluoropropyl-2-beta-carbomethoxy-3beta-(4-iodophenyl) nortropane single-photon emission computed tomography images in the assessment of patients with parkinsonian syndromes. Nucl Med Commun 30:194–201
Badiavas K, Molyvda E, Iakovou I et al (2011) SPECT imaging evaluation in movement disorders: far beyond visual assessment. Eur J Nucl Med Mol Imaging 38:764–773
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 39 kb)
Rights and permissions
About this article
Cite this article
Ueda, J., Yoshimura, H., Shimizu, K. et al. Combined visual and semi-quantitative assessment of 123I-FP-CIT SPECT for the diagnosis of dopaminergic neurodegenerative diseases. Neurol Sci 38, 1187–1191 (2017). https://doi.org/10.1007/s10072-017-2936-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2936-3