Abstract
We conducted a meta-analysis to summarize available evidence regarding the relation between saturated fatty acid (SFA) intake and stroke risk. We searched multiple electronic databases through February 2016. Log relative risks (RRs) with 95 % confidence intervals (CIs) of the highest versus the lowest for cohort studies were weighed by the inverse variance method to obtain combined RRs. 15 prospective studies including 476,569 individuals and 11,074 strokes were included. Higher SFA intake was associated with reduced overall stroke risk [RR = 0.89 (95 % CI 0.82–0.96)] and fatal stroke risk [RR = 0.75 (95 % CI 0.59–0.94)]. Subgroup analysis indicated that higher SFA intake was associated with reduced stroke risks for East-Asians [RR = 0.79 (95 % CI 0.69–0.90)], for dose <25 g/day [RR = 0.81 (95 % CI 0.71–0.92)], for males [RR = 0.85 (95 % CI 0.75–0.96)], and for individuals with body mass index (BMI) <24 [RR = 0.75 (95 % CI 0.65–0.87)], but not for non East-Asians, females, and individuals with dose ≥25 g/day and BMI ≥24. This meta-analysis reveals that higher SFA intake is inversely associated with risk of stroke morbidity and mortality with race, sex, and BMI as key factors influencing this risk. There seems to be a threshold of SFA intake for inverse relation of SFA intake with stroke. However, the stroke-reducing or -increasing effects for specific subtypes and specific food sources of SFA can be concealed. Functions of specific subtypes of SFA (e.g. lignoceric acid) and specific food sources of SFA (i.e. plant vs. animal) in relation to stroke need to be clarified in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saturated fatty acids (SFAs) have no double bonds. A reduction in SFA was regularly recommended as a key intervention to reduce incidence and mortality of cardiovascular disease (CVD). However, a recent meta-analysis [1] indicates that SFA intake has no association with coronary disease, and another meta-analysis [2] including eight prospective studies suggests no association between SFA and stroke risk. Some evidence [3] has found that all SFAs raise high-density lipoprotein cholesterol (HDL-C). In recent years, several large prospective studies [4–9] were added and reported inconsistent results of SFA intake in relation to stroke risk. For instance, four of these studies [5–8] suggest no associations between SFA intake and stroke risk, while one study [4] suggests SFA intake is inversely associated with intraparenchymal hemorrhage and ischemic stroke, and another study [9] indicates SFA intake is inversely associated with deep intraparenchymal hemorrhage and lacunar infarction. Although one recent meta-analysis [10] indicates that SFA intake is not associated with ischemic stroke, there is still lacking the summary evidence on total stroke and hemorrhagic stroke. In addition, the pivotal factors such as fatal stroke risk and body mass index (BMI) were not evaluated. Thus, there is a requirement to clarify the roles of SFA in relation to stroke risk. Here, we conducted a meta-analysis of prospective cohort studies to clarify the associations between SFA intake and stroke risk based on the epidemiologic characteristics of study population.
Methods
Search strategy and selection criteria
References for this meta-analysis were identified through searches of PubMed, Embase, and Web of Knowledge for studies of SFA with the search terms: (“fat” OR “fatty acids”) combined with (“stroke” OR “cerebrovascular disease” OR “cerebrovascular accident” OR “cerebrovascular event” OR “TIA” OR “transient ischemic attack”). Reference lists of retrieved studies and review articles were hand searched to identify any eligible resources. There were no language restrictions. We included studies that met the following criteria (1) a prospective cohort design was used; (2) relative risks (RRs) and their corresponding 95 % confidence intervals (CIs) of stroke relating to SFA intake were provided; (3) covariates (such as alcohol, smoking, and blood pressure) were controlled in the multivariate analysis; and (4) only the most recent publication, or the one with the longest follow-up period, was included when duplicate reports based on the same cohort occurred. We excluded articles that met the following criteria: (1) non prospective cohort study design was used, (2) reviews, (3) non-human studies, and (4) conference abstracts or reports lacking of RRs and the corresponding 95 % CIs of stroke relating to SFA intake.
Data extraction
After the initial assessment for eligibility, P.F.C. and W.H.S. independently completed a data extraction form. Any disagreement about the data was resolved by a third reviewer (M.L.L). We extracted the following information from the selected publications: first author’s name, publication year, cohort name, country, age, sex, size of study population, number of stroke events, methods for saturated fat intake assessment, BMI, length of follow-up, levels of SFA intake, outcome assessment (fatal or non-fatal), hazard ratios (HRs) or RRs of stroke and corresponding 95 % CIs for SFA intake, and covariates adjusted in the statistical analysis. The Newcastle-Ottawa Scale [11] was used for the study quality assessment in this meta-analysis, and we defined a high-quality study as a study with ≥7 awarded stars.
Statistical analysis
For calculating the risk of stroke morbidity and mortality, log RRs of the highest versus the lowest for cohort studies were combined by an inverse variance method. RRs for association of SFA intake and stroke that were often differently reported by each study (such as per-unit or per−1-SD change or comparing quintiles, quartiles, thirds, and other groupings) were transformed, using methods previously described [1]. For obtaining more robust RRs of stroke for SFA intake, we referred to one previous meta-analysis, [2] and RRs with max multivariates adjusted were extracted for each study. In addition, we retrieved all supplemental files of the included studies for RRs of total stroke, specific types of stroke, and specific sex of stroke if available. Statistical heterogeneity was evaluated with Q and I 2 statistics [12]. An I 2 of 25, 50, and 75 % represented low, moderate, and high heterogeneity, respectively. We used the fixed effects model for statistics when I 2 < 50 %; otherwise, we used the random effects model for calculating pooled RRs of stroke for SFA intake.
We performed analyses stratified by length of follow-up, race, dose, sex, stroke type, BMI, score of study quality, and dietary assessment methods. Sensitivity analysis was conducted to detect the potential bias of studies in this meta-analysis. Evidence of publication bias was assessed using funnel plots and the Egger’s test. Data analysis was performed with STATA software package (version 12.0) (StataCorp, College Station, TX, USA), and P < 0.05 was considered as statistically significant.
Results
Study characteristics
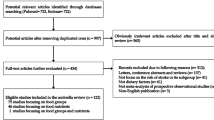
1828 records were identified through database searching, and 2 records [13, 14] were retrieved from a previous meta-analysis. After screening the abstracts, 140 qualified for full review (Fig. 1). Finally, 15 prospective studies [4–9, 13–21] were included (two studies [7, 22] were based on the same cohort, the one [7] with the longest follow-up period was included in this meta-analysis), published between 1984 and 2013 including 11,074 stroke events among 476,569 participants. Five studies were from USA, [8, 13, 15, 17, 18], five from Japan, [4, 9, 16, 19, 20], two from Sweden, [6, 7], one from Israel, [14], one from United Kingdom, [5], and one from Greece [21]. Age ranged from 20 to 89 years. Number of participants ranged from 832 to 87,025, and the number of stroke events ranged from 72 to 3192. Three studies [13, 15, 19] used 24-h recall method, Eleven studies [4–6, 8, 9, 14, 16–18, 20, 21] used food frequency questionnaire (FFQ), and one study [7] used diet history method to evaluate dietary intake of SFA. Average length of follow-up ranged from 7.6 to 23 years. The average dietary SFA intake available in the high categories ranged between 15.4 and 24.9 g/day among East Asian cohorts and 22.1 and 36 g/day among non East Asian cohorts (Table 1). RRs of total stroke, intraparenchymal haemorrhage, and ischaemic stroke with max multivariate adjusted for two included studies [4, 9] were retrieved from the “Results” section rather than routinely from the data sources of tables, with the permission of Boards of Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC); and Japan Public Health Center-based Prospective Study (JPHC) unpublished RRs of subarachnoid hemorrhage with max multivariate adjusted were provided by authors of these two studies. RRs of total stroke, specific type of stroke, and specific sex of stroke were retrieved from the supplemental files of two included studies [7, 19]. All studies report RRs adjusted for age, hypertension or measured blood pressure; two studies [13, 16] did not report RRs adjusted for diabetes; and only one study [16] did not report RRs adjusted for smoking. Of these 15 studies, 12 studies [4–9, 14, 15, 17–19, 21] were awarded as lower quality studies, while three studies [13, 16, 20] were awarded as high quality studies.
Primary outcome
Higher SFA intake was associated with a reduced overall stroke risk [0.89 (95 % CI 0.82–0.96)] (Fig. 2) and a fatal stroke risk [0.75 (95 % CI 0.59–0.94)] (Table 2).
Secondary outcome
Subgroup analysis indicated that higher SFA intake was positively associated with reduced stroke risks for follow-up duration ≥14 years [RR = 0.77 (95 % CI 0.68–0.88)], for East-Asians [RR = 0.79 (95 % CI 0.69–0.90)], for dose <25 g/day [RR = 0.81 (95 % CI 0.71–0.92)] (Fig. 3), for males [RR = 0.85 (95 % CI 0.75–0.96)], for ischemic stroke [RR = 0.90 (95 % CI 0.82–0.99)], for hemorrhagic stroke [RR = 0.76 (95 % CI 0.62–0.92)], for BMI <24 [RR = 0.75 (95 % CI 0.65–0.87)], for quality score ≥7 stars [RR = 0.89 (95 % CI 0.82–0.96)], and for FFQ [RR = 0.91 (95 % CI 0.83–0.99)]. However, higher SFA intake demonstrated no associations with stroke risk for follow-up duration <14 years [RR = 0.95 (95 % CI 0.87–1.04)], for non East-Asians [RR = 0.94 (95 % CI 0.86–1.02)], for dose ≥25 g/day [RR = 1.02 (95 % CI 0.89–1.15)], for females [RR = 1.04 (95 % CI 0.91–1.18)], for BMI ≥24 [RR = 0.94 (95 % CI 0.84–1.04)], for quality score <7 stars [RR = 0.90 (95 % CI 0.66–1.24)], and for 24 h recall [RR = 0.76 (95 % CI 0.51–1.14)] (Table 2).
Publication bias
The statistical analysis indicated that there was no publication bias for intake of SFA in relation to stroke risk according to the Egger’s test (P = 0.618), and the funnel plots were basically symmetrical by visual inspection (Fig. 4a).
Sensitivity analysis
The results of sensitivity analysis demonstrated that higher SFA intake was positively associated with a reduced stroke risk when omitting each study at a time (Fig. 4b).
Discussion
This meta-analysis identified 15 prospective studies [4–9, 13–21] including 476,569 individuals and 11,074 stroke events, indicating that higher SFA intake is inversely associated with risk of overall stroke, stroke mortality, ischaemic stroke, and haemorrhagic stroke, although one previous meta-analysis [2] including eight prospective studies suggests no association between SFA and stroke risk. The potential biological mechanisms of reduced stroke risks by higher intake of SFA are complicated. All SFAs raise HDL-C, with carbohydrate consumption as the reference, lauric (12:0), myristic (14:0), and palmitic (16:0) acid raise total cholesterol and LDL-C, whereas stearic acid (18:0) does not [3]. There is evidence showing that the elevation of baseline triglyceride levels is positively associated with the incidence of stroke, [23] while increased HDL-C levels are associated with reduced risk of ischemic stroke [24]. Thus, based upon our results, individuals seem to benefit more from the HDL-C raising effects of higher SFA intake.
Some recent studies [25–27] demonstrated that total very-long chain SFAs in plasma were associated with decreased risks of coronary heart disease and diabetes (main risk factors for stroke); another study [28] indicated that higher levels of circulating palmitic acid (16:0) were associated with a higher risk of AF, while higher levels of circulating stearic acid (18:0), arachidic acid (20:0), behenic acid (22:0), and lignoceric acid (24:0) were each associated with a lower risk of AF. Very long-chain SFAs (saturated fats with a chain length of 20 or more carbon atoms) are derived from dietary sources, such as peanuts, macadamia nuts, and canola oil, and may also be produced endogenously [29, 30]. Some short-term feeding trials have shown that dietary intake of macadamia nuts increase the circulating levels of arachidic and behenic acids [31] and dietary intake of peanut butter can increase circulating behenic and lignoceric acids [32]. A recent meta-analysis [33] including nine independent prospective cohorts indicated that nut consumption is inversely associated with risk of stroke. Very long-chain SFAs are known to modulate retinal function, anti-inflamatory reactions, [34], and peroxisome-related functionality in the etiology of CVD [35]. Unfortunately, the included studies in this meta-analysis did not report the association between specific subtype of SFA and stroke risk. Thus, the stroke-reducing or stroke-increasing effects for specific subtype of SFA can be concealed.
The background SFA intake was about 12 % (range 9–16 %) in North American studies, while it was about 9 % (range 5–14 %) in Asian studies, and consistently <7 % in Japanese cohorts [9, 10]. For this meta-analysis, the average dietary SFA intake available in the high categories ranged between 15.4 and 24.9 g/day among Japanese cohorts, 22.1 and 36 g/day among non East-Asian cohorts, and 26.1 and 36 g/day among North American cohorts. Based on our results of dose subgroup, there seems to be a threshold of SFA intake (less than 25 g/day; however, the accuracy of this value still needs to be confirmed) for inverse relation of SFA intake with stroke. Compared with other industrialized countries, however, Japan consumes low amounts of fat from animal sources [36]. One Greece cohort study [21] included in this meta-analysis was based on traditional Mediterranean diet, which is characterized by high consumption of legumes, nuts, cereals, vegetables and fruits; moderate-to-high consumption of fish; low consumption of dairy products and meat. Thus, the effect of SFA might not be uniform across ethnic populations, intake levels, or possibly food sources. Further studies are needed to evaluate the association between specific food sources of SFA (i.e. plant vs. animal) and risk of stroke.
In the subgroup analysis, participants with longer duration of SFA intake demonstrated a reduced risk of stroke, and studies with a higher quality score provided homogeneous results. Sex, [37] and BMI [38] are factors influencing the incidence of stroke; our subgroup analysis indicated that higher SFA intake was associated with reduced stroke risk for males and for individuals with lower BMI. Our results demonstrated that higher SFA intake was associated with reduced stroke risk for FFQ subgroup. Three studies [13, 15, 19] included in this meta-analysis used 24 h recall method, which is relatively easy to collect, but the information may not well reflect long-term dietary patterns. However, most included (eleven) studies [4–6, 8, 9, 14, 16–18, 20, 21] in this meta-analysis used FFQ, which can assess long-term diets. Sensitivity analysis demonstrates that high SFA intake is persistently associated with a reduced stroke risk when omitting each study in turn. The heterogeneity between studies in this meta-analysis was at a low level (I 2 = 37.4 %). When we conducted the sex subgroup analysis, the heterogeneity decreased to zero in both male and female subgroups. Thus, sex is considered to be a source of heterogeneity.
Study limitations: first, one limitation inherited in observational studies is the possibility of differing degrees of adjustment for potential confounders. Although majority of studies adjusted for known risk factors for stroke, such as age, smoking, hypertension or measured blood pressure, diabetes, and other dietary factors, the possibility of residual confounding cannot be excluded. Second, this meta-analysis included East-Asians and non East-Asians; the upper limit of normal BMI for Asian populations is 23 kg/m2, and an upper limit of normal BMI issued by World Health Organization guidelines is 25 kg/m2 [39]; in the stratification analysis for BMI, for obtaining more conservative results, we used a midpoint of BMI (24) for subgroup dividing to minimize the bias of observational results.
Conclusion
This meta-analysis of prospective studies reveals that higher SFA intake is inversely associated with risk of stroke morbidity and mortality with race, sex, and BMI as key factors influencing this risk. There seems to be a threshold of SFA intake for inverse relation of SFA intake with stroke. However, the stroke reducing or increasing effects for specific subtypes and specific food sources of SFA can be concealed. Functions of specific subtypes of SFA (e.g. lignoceric acid) and specific food sources of SFA (i.e. plant vs. animal) in relation to stroke need to be clarified in further studies. This meta-analysis adds to recent high-profile scientific evidences that have called for a re-evaluation of dietary guidelines for saturated fat intake and a re-appraisal of the effects of saturated fat on health.
References
Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L et al (2014) Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med 160(6):398–406
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM (2010) Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr 91(3):535–546
Micha R, Mozaffarian D (2010) Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids 45(10):893–905
Yamagishi K, Iso H, Yatsuya H, Tanabe N, Date C, Kikuchi S et al (2010) Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: the Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am J Clin Nutr 92(4):759–765
Atkinson C, Whitley E, Ness A, Baker I (2011) Associations between types of dietary fat and fish intake and risk of stroke in the Caerphilly Prospective Study (CaPS). Public Health 125(6):345–348
Larsson SC, Virtamo J, Wolk A (2012) Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis 221(1):282–286
Wallstrom P, Sonestedt E, Hlebowicz J, Ericson U, Drake I, Persson M et al (2012) Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmo Diet and Cancer Cohort: a prospective study. PLoS One 7(2):e31637. doi:10.1371/journal.pone.0031637
Yaemsiri S, Sen S, Tinker L, Rosamond W, Wassertheil-Smoller S, He K (2012) Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann Neurol 72(5):704–715
Yamagishi K, Iso H, Kokubo Y, Saito I, Yatsuya H, Ishihara J et al (2013) Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: the JPHC Study. Eur Heart J 34(16):1225–1232
de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T et al (2015) Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351:h3978
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558
Mcgee DL, Reed DM, Yano K, Kagan A, Tillotson J (1984) Ten-year incidence of coronary heart disease in the honolulu heart program relationship to nutrient intake. Am J Epidemiol 119(5):667–676
Goldbourt U, Yaari S, Medalie JH (1993) Factors predictive of long-term coronary heart disease mortality among 10,059 male Israeli civil servants and municipal employees. Cardiology 82(2–3):100–121
Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA (1997) Inverse association of dietary fat with development of ischemic stroke in men. JAMA 278(24):2145–2150
Seino F, Date C, Nakayama T, Yoshiike N, Yokoyama T, Yamaguchi M et al (1997) Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J Nutr Sci Vitaminol (Tokyo) 43(1):83–99
Iso H, Stampfer MJ, Manson JE, Rexrode K, Hu F, Hennekens CH et al (2001) Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation 103(6):856–863
He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC et al (2003) Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ 327(7418):777–782
Iso H, Sato S, Kitamura A, Naito Y, Shimamoto T, Komachi Y (2003) Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am J Epidemiol 157(1):32–39
Sauvaget C, Nagano J, Hayashi M, Yamada M (2004) Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke 35(7):1531–1537
Misirli G, Benetou V, Lagiou P, Bamia C, Trichopoulos D, Trichopoulou A (2012) Relation of the traditional Mediterranean diet to cerebrovascular disease in a Mediterranean population. Am J Epidemiol 176(12):1185–1192
Leosdottir M, Nilsson PM, Nilsson J-Å, Berglund G (2007) Cardiovascular event risk in relation to dietary fat intake in middle-aged individuals: data from The Malmö Diet and Cancer Study. Eur J Cardiovasc Prev Rehabil 14(5):701–706
Labreuche J, Deplanque D, Touboul P-J, Bruckert E, Amarenco P (2010) Association between change in plasma triglyceride levels and risk of stroke and carotid atherosclerosis: systematic review and meta-regression analysis. Atherosclerosis 212(1):9–15
Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin I-F et al (2001) High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA 285(21):2729–2735
Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB et al (2014) Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endo 2(10):810–818
Lemaitre RN, Fretts AM, Sitlani CM, Biggs ML, Mukamal K, King IB et al (2015) Plasma phospholipid very-long-chain saturated fatty acids and incident diabetes in older adults: the Cardiovascular Health Study. Am J Clin Nutr 101(5):1047–1054
Malik VS, Chiuve SE, Campos H, Rimm EB, Mozaffarian D, Hu FB et al (2015) Circulating very-long chain saturated fatty acids and incident coronary heart disease in US men and women. Circulation 132(4):260–268. doi:10.1161/CIRCULATIONAHA.114.014911
Fretts AM, Mozaffarian D, Siscovick DS, Djousse L, Heckbert SR, King IB et al (2014) Plasma phospholipid saturated fatty acids and incident atrial fibrillation: the Cardiovascular Health Study. J Am Heart Assoc 3(3):e000889
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45(3):237–249
Guillou H, Zadravec D, Martin PG, Jacobsson A (2010) The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog Lipid Res 49(2):186–199
Garg ML, Blake RJ, Wills RB (2003) Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr 133(4):1060–1063
Lam C, Wong D, Cederbaum S, Lim B, Qu Y (2012) Peanut consumption increases levels of plasma very long chain fatty acids in humans. Mol Genet Metab 107(3):620–622
Zhang Z, Xu G, Wei Y, Zhu W, Liu X (2015) Nut consumption and risk of stroke. Eur J Epidemiol 30(3):189–196
Kihara A (2012) Very long-chain fatty acids: elongation, physiology and related disorders. J Biochem 152(5):387–395
Yamazaki Y, Kondo K, Maeba R, Nishimukai M, Nezu T, Hara H (2014) The proportion of nervonic acid in serum lipids is associated with serum plasmalogen levels and metabolic syndrome. J Oleo Sci 63(5):527–537
Kromhout D, Menotti A, Bloemberg B, Aravanis C, Blackburn H, Buzina R et al (1995) Dietary saturated and transfatty acids and cholesterol and 25-year mortality from coronary heart disease: the seven countries study. Prev Med 24(3):308–315
Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA (2009) Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 40(4):1032–1037
Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L (2010) Excess body weight and incidence of stroke meta-analysis of prospective studies with 2 million participants. Stroke 41(5):e418–e426
Choo V (2002) WHO reassesses appropriate body-mass index for Asian populations. Lancet 360(9328):235
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 31300881).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Cheng, P., Wang, J., Shao, W. et al. Can dietary saturated fat be beneficial in prevention of stroke risk? A meta-analysis. Neurol Sci 37, 1089–1098 (2016). https://doi.org/10.1007/s10072-016-2548-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2548-3