Abstract
Although some studies have reported the associations between specific metal element intake and risk of Parkinson’s disease (PD), the associations between specific metal element intake such as iron intake and PD are still conflicted. We aimed to determine whether intake of iron, zinc, and copper increases/decreases the risk of PD. PubMed, Embase, Web of Knowledge, and Google Scholar were searched. We pooled the multivariate-adjusted relative risks (RRs) or odds ratios using random effects. Study quality was evaluated by the Newcastle–Ottawa Scale. Five studies including 126,507 individuals remained for inclusion, pooled RRs of Parkinson’s disease for moderate dietary iron intake was 1.08 (95 % CI 0.61–1.93, P = 0.787), and for high dietary iron intake was (1.03, 95 % CI 0.83–1.30, P = 0.766), respectively. The pooled RRs of Parkinson’s disease for the highest compared with the lowest dietary iron intake were 1.47 (95 % CI 1.17–1.85, P = 0.001) in western population and in males (RR = 1.43, 95 % CI 1.01–2.01, P = 0.041). The pooled RRs of Parkinson’s disease for moderate or high intake of zinc, and copper were not statistically different (P > 0.05). PD increased by 18 % (RR 1.18, 95 % CI 1.02–1.37) for western population by every 10-mg/day increment in iron intake. Higher iron intake appears to be not associated with overall PD risk, but may be associated with risk of PD in western population. Sex may be a factor influencing PD risk for higher iron intake. However, further studies are still needed to confirm the sex-selective effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the most common serious movement disorder in the world [1]. Some evidence suggests that PD is a multifactorial result of age, ethnicity, environmental factors, and genetics [2–6]. Metal elements such as iron, zinc, and copper—which contribute to the function of metalloenzymes that participate in free radical control and antioxidant defense—have been associated with the development of neurodegenerative disorders [7–9]. Some studies have shown that varied iron levels in independent brain areas in PD [10, 11]. Therefore, as the daily dietary intake of metal elements largely depends on meal composition, [12–14] differences in the dietary intake of metal elements may have an effect on the development of PD.
Several studies [15–19] have reported the associations of dietary metal elements, especially ion intake and PD risk with mixed conclusions. For instance, two studies [15, 18] suggest a high intake of iron is related to risk for PD, one study [16] supported no association between dietary iron intake and PD risk for both men and women, one study [19] found a high dietary iron intake increased PD risk in men but not in women, while another study [17] suggests higher intake of iron may be protective against PD [17]. Also, the dose–response relationship for iron intake and PD risk was not analyzed. Giving these facts, there is a requirement to clarify the associations between dietary iron intake and risk of PD. Here, we used a meta-analysis method aiming to evaluate dietary intake of iron, zinc, or copper, and the risk of PD. In addition, dose–response relationship was evaluated.
Methods
Data sources and searches
PubMed, Embase, Web of Knowledge, and Google Scholar were searched without language restrictions. We used the search following search terms: (“iron” OR “zinc” OR “copper” OR “diet*” OR “food” OR “nutrient*”) AND (“Parkinson*” OR “Parkinson’s disease” OR “PD”). Other potential articles were identified by consulting the previous reviews and reference lists of retrieved records.
Study selection
Studies were included if they met the following five criteria: (1) dietary intake of iron, zinc, copper, or selenium was clearly addressed; (2) PD patients were diagnosed by neurologists according to the specific canonical diagnostic criteria. (3) multivariates were controlled with appropriate statistical methods such as age, sex, education, or BMI; and (4) odds ratios (ORs) or relative risks (RRs) and their corresponding 95 % confidential intervals (CIs) were provided.
The exclusion criteria were as follows: (1) reviews; (2) experimental studies; (3) patients with a diagnosis of severe dementia or those who were unable to complete the questionnaire; and (4) PD with mental illness (with the exception of gene-related depressive PD studies, which were included). Two reviewers verified all potentially suitable studies by the aforementioned inclusion and exclusion criteria, and a third author served as an arbitrator of disagreements.
Data extraction and quality assessment
Log relative risks (RRs) for cohort studies or odds ratios (ORs) for case–control studies were extracted from the included studies. The quality of the included studies was evaluated by the Newcastle–Ottawa Scale (NOS), studies achieving seven or more stars were considered to be of higher quality [20–22]. Any disagreement as to study quality or data extraction was resolved by discussion.
Data synthesis
Log RRs for cohort studies or ORs for case–control studies were weighed by the inverse-variance (1–5) method to obtain pooled relative risks. Etminan’s classification method was used to categorize moderate or high dietary intake of iron, zinc, and copper [23]. ‘Moderate intake’ was defined as a pooled effect size of the second, and third quartiles or the second, third, and fourth quintiles, while ‘high intake’ was defined as the fourth quartile or the fifth quintile. We then pooled the ORs and RRs for each metal element by moderate or high intake. One study [15] contained two sets of multivariates’ controlled ORs, one is age, sex, race, smoking, and BMI; the other one is age, sex, race, smoking, and total Kcal; to obtain more conservative results, we used the combined value of these two multivariates’ controlled ORs.
All five studies provided data on dietary intake of iron [15–19] with two case–controlled studies providing data on dietary intake of zinc and copper [17, 18]. Among the included studies, two mentioned that their studies were based on a population from the Group Health Cooperative Health Maintenance Organization (HMO) in western Washington State, USA [18, 19]; thus, we selected the most recent data for our pooled analysis [19].
We pooled the remaining four studies with data on dietary intake of iron [15–17, 19]. An I 2 of 25, 50, and 75 % represented low, moderate, and high heterogeneity, respectively [24]; we used a random effects model for pooling ORs or RRs. One study was on an Eastern Asian population [17], while the other three studies were on a western population [15, 16, 19], so we did a sensitivity analysis to explore whether geography/ethnicity influences dietary iron’s effect on the risk of PD.
Two studies provided relevant ORs or RRs of moderate and high dietary intake of iron and the risk for PD in both men and women [16, 19]. So, we performed separate subgroup analyses on men and women.
We conducted a dose–response meta-analysis using the method proposed by Greenland and Longnecker [25] and Orsini et al. [26] to compute the trend from the correlated log RR estimates across categories. For each study, the median or mean intake of metal elements for each category was assigned to each corresponding RR. When the median or mean intake in each category was not provided, we assigned the midpoint of the upper and lower boundaries in every category as the average intake. If the upper boundary for the highest category was not reported, we assumed that the boundary had the same amplitude as the nearest category. While for the upper exposure category, we used 1.2-fold, its lower limit [27]. Publication bias was assessed using Egger’s test [28]. The analysis was conducted by STATA, version 12 (StataCorp, College Station, TX).
Results
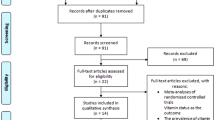
Five studies [15–19] including 126,507 individuals remained for inclusion in this meta-analysis (Fig. 1). The characteristics of the included studies are shown in Table 1: one 16-year cohort study [16] and four case–control studies [15, 17–19]. Four studies [15–17, 19] with data on dietary intake of iron with non-overlapping individuals. Two studies [17, 18] provided data on dietary zinc and copper intake. Two western population-based studies [16, 19] provided dose–response related data on dietary iron. One study [17] scored 9 stars and the other four studies [15, 16, 18, 19] scored 7 stars, all of them were considered as high-quality studies.
Primary outcome
The pooled RRs of Parkinson’s disease for moderate dietary iron intake were 1.08 (95 % CI 0.61–1.93, P = 0.787), and for high dietary iron intake were (1.03, 95 % CI 0.83–1.30, P = 0.766), respectively (Fig. 2; Table 2).
Forest plot of relative risks (RRs) for high intake of iron and PD risk. a Forest plot of relative risks (RRs) for all studies of high iron intake and PD risk. The overall RR was not statistically significant (P = 0.787). b Forest plot of relative risks (RRs) of high iron intake and PD risk for western population. The overall RR was statistically significant (P = 0.001). c Forest plot of relative risks (RRs) for the men subgroup of high iron intake and PD risk. The overall RR was statistically significant (P = 0.041)
Secondary outcome
The pooled RRs of Parkinson’s disease for the highest compared with the lowest dietary iron intake in western population were (RR = 1.47, 95 % CI 1.17–1.85, P = 0.001) (Fig. 2; Table 3). The pooled RRs of Parkinson’s disease for moderate or high intake of zinc, and copper are shown in Table 3. The pooled RRs of PD for high dietary iron intake in men were 1.43 (95 % CI 1.01–2.01, P = 0.041), while those in women were 1.22 (95 % CI 0.89–1.66, P = 0.217) (Fig. 2; Table 3).
Dose–response relationship
Linear dose–response analysis indicated a positive association between iron intake and PD risk (P = 0.028); PD increased by 18 % (RR 1.18, 95 % CI 1.02–1.37) for every 10-mg/day increment in iron intake. Non-linear dose–response analysis also indicated a positive association between iron intake and PD risk (P < 0.01) (Fig. 3).
Dose–response relationship for iron intake and PD risk in western population. The solid line and long dash line represent the estimated relative risk and its 95 % confidence interval. Short dash line represents the linear relationship. PD increased by 18 % (RR 1.18, 95 % CI 1.02–1.37) for western population by every 10-mg/day increment in iron intake
Publication bias
There was no significant publication bias for either moderate intake of iron and PD risk (P = 0.255) or high intake of iron and PD risk (P = 0.302).
Discussion
Metal ions such as iron, zinc, and copper are essential trace elements responsible for the function of many cellular enzymes and proteins; however, the same elements become toxic in the event of excessive intracellular accumulation. [29] The zinc, copper, and iron levels in the brain are stringently regulated by the transport of metal ions across the blood–brain barrier (BBB), and their dysregulation has been increasingly implicated in interactions with the key protein actors in several neurodegenerative diseases [7, 30, 31]. For example, previous evidence [32] suggests the elevated iron levels in the basal ganglia of PD patients as well as decreased copper and increased zinc levels in the substantia nigra of PD patients.
This meta-analysis including five studies was based on a large scale of population, the results revealed that a moderate or high intake of iron displays no association with the risk of developing PD. However, through a subgroup analysis, high intake of iron was positively associated with the risk of PD in western population (RR = 1.47, 95 % CI 1.17–1.85, P = 0.001). There is evidence [33] suggesting that the incidence rate of PD for non-Hispanic Caucasians is higher compared with Asians. Based on our results, geography/ethnicity may be an influencing factor on iron intake’s effect on PD risk. As a result of this subgroup analysis, the I 2 for the overall relative risk for high iron intake was originally 82.4 and decreased to zero, so we consider this population-based difference may be attributable for this high heterogeneity. In addition, no publication bias was found between the included studies.
There is evidence [33] showing that the incidence rate of PD is greater in men than in women. Our sex subgroup result suggests that high intake of iron is positively associated with the risk of PD in men (RR = 1.43, 95 % CI 1.01–2.01, P = 0.041), but not associated with the risk of PD in women. Menstrual blood loss is an important factor influencing iron status in women [34, 35]. Thus, based upon our results, sex seems to be a factor influencing iron intake and PD risk. According to the result of our meta-analysis, dose–response meta-analysis indicated that a 10-mg/day increment in dietary iron intake was associated with a 18 % augmentation in PD risk for the western population.
Study limitations: First, smoking history, coffee drinking, and alcohol [36] are all important factors influencing PD risk. However, when we confined these multivariates, the results remained persistent. Second, there was only one East Asian population study [17] showing that higher iron intake reduces the risk of PD; thus, the study power in this population was limited without pooling. Third, we did not find any data on the serum concentrations of iron or any other metal element in the included studies, so we could not analyze any possible associations between serum concentrations of metal elements and the risk of PD. Fourth, the interpretation for the results should be cautious, although the results showed that higher dietary iron intake is associated with stroke risk for men. However, in the light of limited power, the differences by sex in the results may well represent random variation. Fifth, only two studies provided copper- and zinc-related data. Hence, the results still need to be further confirmed. Sixth, there are exposure assessment limitations of individual studies, because four studies used food frequency questionnaire (FFQ) and one study used diet history questionnaire (DHQ) to estimate the metal elements’ intake. Hence, there may exist potential recall bias.
Conclusion
Higher iron intake appears to be not associated with overall PD risk, but may be associated with risk of PD in western population. Sex may be a factor influencing PD risk for higher iron intake. However, further studies are still needed to confirm the sex-selective effects.
References
Samii A, Nutt JG, Ransom BR (2004) Parkinson’s disease. Lancet 363:1783–1793
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50:1346–1350
Kamel F (2013) Epidemiology. Paths from pesticides to Parkinson’s. Science 341:722–723
Steece-Collier K, Maries E, Kordower JH (2002) Etiology of Parkinson’s disease: genetics and environment revisited. Proc Natl Acad Sci USA 99:13972–13974
Trinh J, Farrer M (2013) Advances in the genetics of Parkinson disease. Nat Rev Neurol 9:445–454
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA et al (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Cheml Biolo 12:222–228
Fang Y-Z, Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18:872–879
Machlin LJ, Bendich A (1987) Free radical tissue damage: protective role of antioxidant nutrients. FASEB J 1:441–445
Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA et al (2007) Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68:1820–1825
Yu X, Du T, Song N, He Q, Shen Y, Jiang H et al (2013) Decreased iron levels in the temporal cortex in postmortem human brains with Parkinson disease. Neurology 80:492–495
Hunt JR (2003) Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 78:633s–639s
Lee HP, Zhu X, Liu G, Chen SG, Perry G, Smith MA et al (2010) Divalent metal transporter, iron, and Parkinson’s disease: a pathological relationship. Cell Res 20:397–399
Sandstrom B, Arvidsson B, Cederblad A, Bjorn-Rasmussen E (1980) Zinc absorption from composite meals. I. The significance of wheat extraction rate, zinc, calcium, and protein content in meals based on bread. Am J Clin Nutr 33:739–745
Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL (1999) Adult nutrient intake as a risk factor for Parkinson’s disease. Int J Epidemiol 28:1102–1109
Logroscino G, Gao X, Chen H, Wing A, Ascherio A (2008) Dietary iron intake and risk of Parkinson’s disease. Am J Epidemiol 168:1381–1388
Miyake Y, Tanaka K, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y et al (2011) Dietary intake of metals and risk of Parkinson’s disease: a case–control study in Japan. J Neurol Sci 306:98–102
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H (2003) Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 60:1761–1766
Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr, Swanson PD, Checkoway H (2009) Dietary fats, cholesterol and iron as risk factors for Parkinson’s disease. Parkinsonism Relat Disord 15:47–52
Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D (2006) Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry 63:530–538
Stang A (2010) Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M (2015) The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford_web.ppt. Accessed Apr 12 2015
Etminan M, Gill SS, Samii A (2005) Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol 4:362–365
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose–response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata J 6:40
Berlin JA, Longnecker MP, Greenland S (1993) Meta-analysis of epidemiologic dose–response data. Epidemiology 4:218–228
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629-634
Kozlowski H, Janicka-Klos A, Brasun J, Gaggelli E, Valensin D, Valensin G (2009) Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coord Chem Rev 253:2665–2685
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 106:1995–2044
Kozlowski H, Luczkowski M, Remelli M, Valensin D (2012) Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord Chem Rev 256:2129–2141
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(Pt 4):1953–1975
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA et al (2003) Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol 157:1015–1022
Blanco-Rojo R, Toxqui L, López-Parra AM, Baeza-Richer C, Pérez-Granados AM, Arroyo-Pardo E et al (2014) Influence of diet, menstruation and genetic factors on iron status: a cross-sectional study in Spanish women of childbearing age. Int J Mol Sci 15:4077–4087
Coad J, Conlon C (2011) Iron deficiency in women: assessment, causes and consequences. Curr Opin Clin Nutr Metab Care 14:625–634
Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ et al (2012) Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 72:893–901
Acknowledgments
We thank the scientific editors at Impactys (www.impactys.com) for editing and proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
P. F. Cheng and J. Yu contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Cheng, P., Yu, J., Huang, W. et al. Dietary intake of iron, zinc, copper, and risk of Parkinson’s disease: a meta-analysis. Neurol Sci 36, 2269–2275 (2015). https://doi.org/10.1007/s10072-015-2349-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2349-0