Abstract
The objective of this study was to explore the efficacy of glial fibrillary acidic protein (GFAP) in differentiating intracerebral hemorrhage (ICH) from ischemic stroke (IS). Suspicious patients of acute stroke were screened and finally diagnosed by computed tomography and magnetic resonance imaging. Blood samples were collected within 2–6 h after onset of symptoms, and serum GFAP level was determined by ELISA assay. The functional outcome for the patients was determined by modified Rankin Scale (mRS) 90 days after onset of symptoms. 43 ICH patients and 65 IS patients were enrolled. GFAP concentration in ICH group was significantly higher than in IS group (p < 0.001). Significant correlation was found when comparing GFAP with National Institutes of Health Stroke Scale (NIHSS) (r = 0.418, p = 0.005) and hemorrhage volume (r = 0.840, p < 0.001) in ICH group, while such correlation was not observed in IS group. ROC analysis indicated that GFAP level at the cut-point of 0.7 ng/ml yielded an AUC of 0.901 (95 % CI 0.828–0.950) with high sensitivity (86.0 %) and specificity (76.9 %) to differentiate ICH from IS. Patients with higher serum GFAP concentration in ICH group experienced poorer functional disability (r = 0.755, p < 0.001), while this phenomenon was not observed in IS group (r = −0.114, p = 0.368). ROC curve analysis found that GFAP level at the cut-point of 1.04 ng/ml yielded an AUC of 0.936 (95 % CI 0.817–0.988) in identifying patients with poor functional outcome, at the sensitivity and specificity of 95.7 and 80.0 %, respectively. GFAP test is a promising technique for diagnosis of ICH from IS and prediction of short-term functional outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been reported that intracerebral hemorrhage (ICH) accounts for 8–15 % of all strokes in the USA, Europe, and Australia [1, 2], and the rate in Asia is about two-fold to three-fold higher (20–30 %) [3, 4]. After the incidence of ICH, 37–47 % of the patients may die within the first year and a large portion of the survivors may suffer serious neurological deficits [5, 6]. Unfortunately, current treatment for ICH lags far behind those for ischemic stroke (IS) [4].
Nowadays, brain imaging remains the gold standard to differentiate ICH and IS [7]. However, even though computed tomography could be easily performed in hospitals, the diagnostic accuracy largely depends on the experience of the clinicians [8, 9]. Moreover, no diagnosis-specific measures can be utilized in the prehospital setting, resulting in a delay of the treatment [10]. Thus, the delay in the diagnosis will certainly increase the rate of morbidity and mortality as well as the treatment costs for the patients. A simple diagnostic test applicable in the prehospital setting may facilitate primary intervention and subsequently benefit the patients.
Searching for ideal stroke biomarkers that can be used in the diagnosis has never stopped. Until now, the most promising biomarker is glial fibrillary acidic protein (GFAP) [11, 12], which acts as a highly brain-specific intermediate filament protein and plays an important role in maintaining the structural integrity of astroglial cells [13]. Human cells do not secret GFAP under normal physiological conditions and thus GFAP cannot be detected in blood samples of healthy individuals [14]. However, when astroglial cells and blood–brain barrier are disrupted, the GFAP molecules will release in cerebrospinal fluid and blood and become detectable. It has been reported that the time window in 2–6 h after stroke onset is optimal for using the GFAP test to distinguish ICH from IS [15]. This is supported by previous studies, but most evidence was obtained in Europe [15–18]. Application of GFAP test for ICH diagnosis in clinical practice needs more reliable evidence.
We therefore prospectively collected the blood samples from stroke patients and investigated the efficacy of using the GFAP test in differentiating diagnosis of ICH and IS and identifying ICH patients with poor short-term functional outcome.
Patients and methods
The research was performed in accordance with the Declaration of Helsinki. The approval by the ethic committee of our institution, and the informed consents by the patients were obtained before starting the study.
Patients
From February 2013 to November 2014, suspicious patients with symptoms of acute stroke were screened in our center. The inclusion criteria were as follows: (a) 2–6 h from symptom onset to hospital admission and (b) definite diagnosis of ICH or IS by initial or consecutive computed tomography or magnetic resonance imaging of brain. Patients who met the following criteria were excluded: (a) negative findings in repeated brain imaging (computed tomography or magnetic resonance imaging) and (b) with a history of brain injury, ICH, IS, brain tumor, renal failure, and any other diseases on the central nervous system.
The severity of the neurological deficits at admission was evaluated using the National Institutes of Health Stroke Scale (NIHSS), and the volume of hemorrhage in ICH was assessed by the standard ellipsoid method [19]. Infarct size in the anterior circulation was categorized into three degree: <1/3 of the middle cerebral artery (MCA) territory, 1/3–2/3 of the MCA territory, and >2/3 of the MCA territory. The infarct size in posterior circulation was not categorized due to limited cohort. The functional disability caused by stroke was rated using the modified Rankin Scale (mRS) 90 days after the onset of symptoms. According to mRS, patients were classified into mild disability with self-care ability (mRS 0–2), mild disability without self-care ability, or death (mRS 3–5). Baseline characteristic data of the patients, such as age, gender, and time span from symptoms onset to hospital admission, were also collected.
Blood samples and enzyme-linked immune sorbent (ELISA) analysis
3 ml fasting venous blood was collected from each patient at hospital admission and immediately centrifuged at 2000g for 10 min. The serum was collected and stored at −80 °C until further analysis. 100 µl of the samples was used for determination of the serum GFAP concentration with a commercial ELISA kit (CSB-E08601 h, Cusabio, Wuhan, China) by a blinded coworker. Each reaction was conducted in triplicate.
Treatment
All patients were treated in accordance with Chinese guidelines on ICH and IS. The conservative management included basic life monitoring and support, maintenance of temperature, blood pressure and glucose for both ICH and IS patients, and control of intracranial pressure and hemostatic for ICH patients and antiplatelet agent and heparin products for IS patients. Surgical procedures were conducted in selected ICH patients, while thrombolysis management was conducted for selected IS patients. Besides, clinical management of the patients was determined by the responsible doctors and not intervened by the results of the present study.
Statistical analysis
SPSS 13.0 software package (SPSS Inc., Chicago, USA) was used for statistical analysis. p < 0.05 was considered statistically significant. Comparison between the groups was carried out using Student’s t test, and Mann–Whitney U test was used to analyze enumeration data.
Spearman’s rank correlation analysis was used to evaluate the correlation between GFAP level and NIHSS, mRS in both the ICH and IS groups, or infarct size of anterior circulation in the IS group. Pearson’s correlation analysis was utilized to assess the correlation between GFAP level and hemorrhage volume in the ICH group. Receiver-operating characteristic (ROC) curve analysis and the area under the curve (AUC) were applied to determine the efficacy of using GFAP test to differentiate ICH from IS and identify the ICH patients with poor functional disability.
Results
Herein, 43 ICH patients and 65 IS patients were enrolled. As shown in Table 1, there was no significant difference between the ICH and IS groups in terms of gender (p = 0.782), the time from symptoms onset to hospital admission (p = 0.336), the number of patients with hypertension (p = 0.896) and diabetes (p = 0.890), and NIHSS (p = 0.975). 2 patients in the ICH group received surgical treatment, and 11 patients in the IS group underwent thrombolysis management. Details of nidus in both the groups are also presented in Table 1.
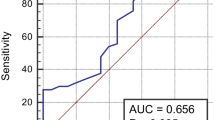
The serum concentration of GFAP in the ICH group was significantly higher than in the IS group (p < 0.001, Fig. 1a). In the ICH group, significant correlation was found between GFAP level and NIHSS (r = 0.418, p = 0.005, Fig. 2a) or hemorrhage volume (r = 0.840, p < 0.001), while Spearmen’s correlation analysis indicates no significant correlation between GFAP level and NIHSS (r = 0.223, p = 0.74, Fig. 2b) or infarct size (r = −0.039, p = 0.768) in the IS group. The ROC analysis indicates that GFAP level at the cut-point of 0.7 ng/ml yielded an AUC of 0.901 (95 % CI 0.828–0.950) with the sensitivity of 86.0 % and specificity of 76.9 % in the differential diagnosis of ICH and IS (Fig. 3a).
Comparison of GFAP level among the different groups. a Serum concentration of GFAP in the ICH group is significantly higher than in the IS group (1.6 ± 0.8 vs. 0.6 ± 0.4 ng/ml). b Serum concentration of GFAP is significantly different between patients with favorable and poor functional outcomes in the ICH group (0.7 ± 0.4 vs. 1.2 ± 1.0 ng/ml)
Functional outcome assessed by mRS after 3 months was favorable in 20 patients and poor in 23 patients in the ICH group, while for the IS group, the results were 36 favorable patients and 29 poor patients (p = 0.369). Serum concentration of GFAP was significantly different between the patients with favorable and poor functional outcome in the ICH group (p = 0.001, Fig. 1b). The functional ability was loosen to a much higher extent for the patients with higher serum GFAP concentration (r = 0.755, p < 0.001) in the ICH group, but similar phenomenon was not observed in the IS group (r = −0.114, p = 0.368). Furthermore, ROC analysis was conducted to explore the efficacy of using the GFAP test to predict patients’ functional disability in the ICH group. It was found that the GFAP level at the cut-point of 1.04 ng/ml yielded an AUC of 0.936 (95 % CI 0.817–0.988) for identification of the patients with poor functional outcome, and the sensitivity and specificity were 95.7 and 80.0 %, respectively (Fig. 3b).
Discussion
The present research indicates that GFAP level significantly increased in serum of the ICH patients compared to those with IS 2–6 h after symptoms onset and positively correlated with hemorrhage volume, NIHSS, or mRS in the ICH group. The use of GFAP test can distinguish ICH patients from IS patients and identify the patients with unfavorable functional disability in the ICH group.
Regarding the capability of GFAP test in determination of the type of strokes, the most important aspect is the time point to detect GFAP level in blood. GFAP is hard to be detected in blood of healthy individuals [14]. However, in the process of ICH, the impaired astrocytes and blood–brain barrier lead to a rapid release of GFAP into blood [16, 20]. In contrast, for IS patients, the destruction of astrocytes and blood–brain barrier progresses gradually. The death and lysis of necrotic cells commonly occur in 6–12 h after ischemia [20, 21], and GFAP concentration reaches a peak level in the coming 48–96 h [22, 23]. Dvorak et al. found that there was no significant difference in serum GFAP level between IS and ICH patients within 1 h after stroke onset, and the overall accuracy in distinguishing ICH patients from IS patients was more than 80 % in 2–6 h after stroke onset [15]. Based on these studies, 2–6 h after stroke onset is considered as the optimal time window to determine serum GFAP level for the differential diagnosis [15–18]. However, controversial results have also been reported. A subgroup study showed that the GFAP level for IS patients was also high in less than 60 min after the onset of symptoms [18].
Our study confirmed that GFAP level rapidly elevated in Chinese ICH patients within 2–6 h after stoke onset, while the level was relatively stable in Chinese IS patients during the same period. The ROC curve analysis also indicates that GFAP test could serve as a promising tool to identify ICH patients from IS patients. It was reported that the sensitivity of using GFAP test in detecting ICH patients from IS patients ranged from 79 to 92.3 %, with the specificity ranging from 89.4 to 100 % [15–18]. In the present study, the sensitivity and specificity was 86.0 and 76.9 %, respectively, which was relatively lower than those reported in the previous studies. It is worthy to note that there was a wide range of cut-point (2.9 ng/l–2.0 μg/l) of GFAP level selected for the differentiating diagnosis in these previous studies [10], while in the present study, the cut-point achieving optimal AUC was 0.7 μg/l. The exact explanation for these differences remains unknown. Many factors may be involved, such as race, disease status, test time, and test techniques. Before application of GFAP test in clinical practice, standard determination procedures should be first established.
A positive result obtained by GFAP test alone cannot confirm ICH in acute stoke, and a negative result also cannot rule out the possibility of ICH. Considering the relatively high sensitivity and specificity, GFAP test has the potential to be applied in the differentiating diagnosis and conducting different therapy strategies for ICH and IS patients, especially in the prehospital setting.
The present study showed that serum GFAP concentrations were positively correlated with hemorrhage volume and NIHSS. The clinical symptoms determined by NIHSS in the present study were usually determined by bleeding volume [24]. Thus, the reasonable explanation may be that larger hemorrhage volume leads to more serious damage in the patient and consequently more GFAP will be released. The result is consistent with previous studies [15, 16, 18]. The correlation index between GFAP level and NIHSS was 0.418 in the present study, which was 0.336 in a previous study [16]. The correlation index between GFAP level and hemorrhage volume was 0.840 in this study, while the value was previously reported to be 0.462 [16] and 0.755 [15]. It can be seen that the correlation index between GFAP level and NIHSS is higher than that between GFAP level and hemorrhage volume, which verify a close relation between GFAP level and the destruction of astrocytes and blood–brain barrier from another perspective. On the contrary, it was found that GFAP level did not increase with infarct size and NIHSS in IS patients. As discussed above, the release of GFAP depends on the destruction of astroglial cells and brain–blood barrier. Since such destruction is a gradual process in IS patients, no or only a few GFAP molecules were released into blood in the early stage. Transient perfusion deficit may be induced by thrombolytic therapy or interventional therapy, which may only lead to dysfunction other than structural damage. Under this circumstance, leakage of GFAP is impossible even in a long surveillance. It was reported that there was no significant increase in serum GFAP level when the patient has received successful thrombolytic therapy for middle cerebral artery blockage [25]. With postponed testing time, the correlation between GFAP level and ischemic infarct size is expected to become more obvious. GFAP concentrations obtained 12 h after stroke onset were found to be highly correlated with NIHSS (r = 0.486) and infarct volume (r = 0.563) [26].
Another important discovery of the study is that positive correlation is found between mRS and serum GFAP concentrations, and GFAP at the cut-off point of 1.04 ng/ml has 95.7 % of sensitivity and 80.0 % of specificity in detecting patients with poor short-term functional disability. A previous study indicated that there was a significant correlation between an increase in GFAP concentrations and poor functional outcome for IS patients [26]. It is worthy to note that the maximal correlation was found 96 h after stroke onset, and the interval was obviously longer.
Even though the results are encouraging, there are several limitations needed to be considered. First, all the patients were from a single institution, whether the cohort can represent the whole population needs further investigation. Second, the sample size is limited, and larger scale studies with multiple-centers are needed to confirm the results. Third, elevated plasma GFAP level was also found in other diseases, such as trauma and malignant tumor [27, 28]. The present study only considered acute stroke, and thus, the conclusions in terms of diagnostic sensitivity and specificity are limited. Fourth, the influence of other clinical parameters on GFAP level were not considered, such as drugs, infection, renal function, diabetes, and alcohol intake. Fifth, the role of treatment, which is important for defining further prognosis factors and may affect the GFAP level, was not analyzed in the present study.
Conclusion
This study indicates that performance of GFAP test within 2–6 h after stroke onset could serve as a promising technique in the differentiating diagnosis of ICH and IS. This technique may also be used to evaluate the severity of hemorrhage and predict short-term functional outcome.
References
Sudlow CL, Warlow CP (1997) Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International stroke incidence collaboration. Stroke 28(3):491–499
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344(19):1450–1460
Suzuki K, Kutsuzawa T, Takita K, Ito M, Sakamoto T, Hirayama A et al (1987) Clinico-epidemiologic study of stroke in Akita, Japan. Stroke 18(2):402–406
Kreitzer N, Adeoye O (2013) An update on surgical and medical management strategies for intracerebral hemorrhage. Semin Neurol 33(5):462–467
Juvela S, Hillbom M, Palomaki H (1995) Risk factors for spontaneous intracerebral hemorrhage. Stroke 26(9):1558–1564
Nilsson OG, Lindgren A, Brandt L, Saveland H (2002) Prediction of death in patients with primary intracerebral hemorrhage: a prospective study of a defined population. J Neurosurg 97(3):531–536
Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A et al (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38(5):1655–1711
Schriger DL, Kalafut M, Starkman S, Krueger M, Saver JL (1998) Cranial computed tomography interpretation in acute stroke: physician accuracy in determining eligibility for thrombolytic therapy. JAMA 279(16):1293–1297
Grotta JC, Chiu D, Lu M, Patel S, Levine SR, Tilley BC et al (1999) Agreement and variability in the interpretation of early CT changes in stroke patients qualifying for intravenous rtPA therapy. Stroke 30(8):1528–1533
Zhang J, Zhang CH, Lin XL, Zhang Q, Wang J, Shi SL (2013) Serum glial fibrillary acidic protein as a biomarker for differentiating intracerebral hemorrhage and ischemic stroke in patients with symptoms of acute stroke: a systematic review and meta-analysis. Neurol Sci 34(11):1887–1892
Schiff L, Hadker N, Weiser S, Rausch C (2012) A literature review of the feasibility of glial fibrillary acidic protein as a biomarker for stroke and traumatic brain injury. Mol Diagn Ther 16(2):79–92
Maas MB, Furie KL (2009) Molecular biomarkers in stroke diagnosis and prognosis. Biomark Med 3(4):363–383
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25(9–10):1439–1451
Missler U, Wiesmann M, Wittmann G, Magerkurth O, Hagenstrom H (1999) Measurement of glial fibrillary acidic protein in human blood: analytical method and preliminary clinical results. Clin Chem 45(1):138–141
Dvorak F, Haberer I, Sitzer M, Foerch C (2009) Characterisation of the diagnostic window of serum glial fibrillary acidic protein for the differentiation of intracerebral haemorrhage and ischaemic stroke. Cerebrovasc Dis 27(1):37–41
Foerch C, Curdt I, Yan B, Dvorak F, Hermans M, Berkefeld J et al (2006) Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J Neurol Neurosurg Psychiatry 77(2):181–184
Unden J, Strandberg K, Malm J, Campbell E, Rosengren L, Stenflo J et al (2009) Explorative investigation of biomarkers of brain damage and coagulation system activation in clinical stroke differentiation. J Neurol 256(1):72–77
Foerch C, Niessner M, Back T, Bauerle M, De Marchis GM, Ferbert A et al (2012) Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 58(1):237–245
Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G (1993) Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7):987–993
Brunkhorst R, Pfeilschifter W, Foerch C (2010) Astroglial proteins as diagnostic markers of acute intracerebral hemorrhage-pathophysiological background and clinical findings. Trans Stroke Res 1(4):246–251
Persson L, Hardemark HG, Bolander HG, Hillered L, Olsson Y (1989) Neurologic and neuropathologic outcome after middle cerebral artery occlusion in rats. Stroke 20(5):641–645
Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ (2000) Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 31(11):2670–2677
Niebroj-Dobosz I, Rafalowska J, Lukasiuk M, Pfeffer A, Mossakowski MJ (1994) Immunochemical analysis of some proteins in cerebrospinal fluid and serum of patients with ischemic strokes. Folia Neuropathol 32(3):129–137
Huttner HB, Kohrmann M, Tognoni E, Juttler E, Richter G, Dorfler A et al (2008) Clinical severity predicts time to hospital admission in patients with spontaneous intracerebral hemorrhage. Cerebrovasc Dis 25(6):533–538
Foerch C, du Mesnil R, de Rochemont O, Singer T, Neumann-Haefelin M, Buchkremer FE Zanella et al (2003) S100B as a surrogate marker for successful clot lysis in hyperacute middle cerebral artery occlusion. J Neurol Neurosurg Psychiatry 74(3):322–325
Wunderlich MT, Wallesch CW, Goertler M (2006) Release of glial fibrillary acidic protein is related to the neurovascular status in acute ischemic stroke. Eur J Neurol 13(10):1118–1123
Jung CS, Foerch C, Schanzer A, Heck A, Plate KH, Seifert V et al (2007) Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain 130(Pt 12):3336–3341
Pelinka LE, Kroepfl A, Schmidhammer R, Krenn M, Buchinger W, Redl H et al (2004) Glial fibrillary acidic protein in serum after traumatic brain injury and multiple trauma. J Trauma 57(5):1006–1012
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, L., Yang, Y., Zhang, M. et al. The use of serum glial fibrillary acidic protein test as a promising tool for intracerebral hemorrhage diagnosis in Chinese patients and prediction of the short-term functional outcomes. Neurol Sci 36, 2081–2087 (2015). https://doi.org/10.1007/s10072-015-2317-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2317-8