Abstract
Endovascular treatment (ET) showed to be safe in acute stroke, but its superiority over intravenous thrombolysis is debated. As ET is rapidly evolving, it is not clear which role it may deserve in the future of stoke treatments. Based on an observational design, a treatment registry allows to study a broad range of patients, turning into a powerful tool for patients’ selection. We report the methodology and a descriptive analysis of patients from a national registry of ET for stroke. The Italian Registry of Endovascular Treatment in Acute Stroke is a multicenter, observational registry running in Italy from 2010. All patients treated with ET in the participating centers were consecutively recorded. Safety measures were symptomatic intracranial hemorrhage, procedural adverse events and death rate. Efficacy measures were arterial recanalization and 3-month good functional outcome. From 2008 to 2012, 960 patients were treated in 25 centers. Median age was 67 years, male gender 57 %. Median baseline NIHSS was 17. The most frequent occlusion site was Middle cerebral artery (46.9 %). Intra-arterial thrombolytics were used in 165 (17.9 %) patients, in 531 (57.5 %) thrombectomy was employed, and 228 (24.7 %) patients received both treatments. Baseline features of this cohort are in line with data from large clinical series and recent trials. This registry allows to collect data from a real practice scenario and to highlight time trends in treatment modalities. It can address unsolved safety and efficacy issues on ET of stroke, providing a useful tool for the planning of new trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Intra-arterial revascularization procedures were not demonstrated to improve functional outcome of stroke patients more than intravenous thrombolysis in three recently published randomized controlled trials [1–3], although the intra-arterial approach was at least as safe as intravenous thrombolysis.
As endovascular treatment is an expensive and in non-expert hands a possibly risky procedure, some concerns about the opportunity to carry on this treatment in acute stroke patients have raised in the scientific community. On the other hand, intra-arterial intervention represents the only possibility of treatment for patients not eligible for intravenous thrombolysis, and a possible add-on approach for patients not responding to intravenous treatment. Moreover, endovascular technique is continuously evolving, due to the introduction of new devices and catheters that makes recanalization faster and more effective. For these reasons, it is still not clear nowadays which role the endovascular therapy may deserve in the future of acute stoke treatments.
Aims
Based on an observational study design, with few inclusion and exclusion criteria, a patient registry allows to study a large sample of a broad range of patients, making the results more generalizable [4]. For this reason, a registry of endovascular procedures could be a powerful tool to identify the most suitable patient to be addressed to this treatment and the most effective endovascular approach, also to build a proper clinical trial.
Here, we report the methodology and a descriptive analysis of patients from a multicenter registry of endovascular stroke treatments running in Italy from 2010.
Methods
The Italian Registry of Endovascular Stroke Treatments is a multicenter, prospective, observational internet-based registry (http://www.registroendovascolare.it). Supported by the Italian Ministry of Health, it represents a collaborative effort of Interventional Neuroradiologists and Stroke Neurologists to collect clinical and instrumental data of acute ischemic stroke patients treated with intra arterial thrombolysis/thrombectomy.
The project started in 2010 and was developed in two phases. In the first phase, participants were asked to retrospectively register all stroke patients who had undergone endovascular treatment in their center from January 1, 2008 to December 31, 2010. This phase was used as a run-in approach for the participants and was useful to determine the procedure volume of each center. In the second phase, the registration of procedures was performed in a prospective manner.
Centers selection was based on the concomitant presence of safe implementation of treatments in stroke (SITS) accreditation for intravenous thrombolysis and Neuroradiological expertise in endovascular treatment of stroke, according to a pre-specified form. Both academic and non-academic hospitals were recruited. For each center, one Interventional Neuroradiologist and one Neurologist were involved to assure accuracy of both clinical and instrumental data. They were endowed with a password-protected access to enter anonymous patient data.
All patients treated with intra-arterial thrombolysis/thrombectomy were consecutively recorded. This population included patients who were not candidates for intravenous thrombolysis or in whom intravenous thrombolysis had failed; moreover, patients recruited in ongoing randomized clinical trials including endovascular treatment as a therapeutic option were also recorded. Exclusion criteria for intravenous thrombolysis were recorded, as well as intravenous thrombolytic treatment preceding the procedure.
For each patient, demographics, stroke risk factors, premorbid conditions, stroke severity, baseline CT scan, and non-invasive neurovascular imaging before treatment were collected. Endovascular treatment data were reported both summarized and in details, according to chronological progression of the procedure. Information from clinical and instrumental monitoring was collected during the hospital stay and at discharge. Clinical follow-up was assessed by modified Rankin Scale (mRS) at 3 months.
Symptomatic intracranial hemorrhage (s-ICH) was defined as any intracranial hemorrhage associated with ≥4 point increase at 24 h NIHSS, according to ECASS II definition [5]. S-ICH, procedural adverse events (namely subarachnoid hemorrhage, and vessel dissection) and death rate were considered as safety measures.
For efficacy measures, arterial recanalization assessed by the thrombolysis in cerebral infarction (TICI) scale [6], and good functional outcome defined as mRS 0–2 at 3 months were evaluated.
For each variable, explicit data definition was reported on an instruction manual, available on line, to assure internal validity. Real-time consultation of database was possible by means of preformed queries by each center. Participant physicians regularly received a newsletter about registry’s activities, and a personalized e-mail alert with missing data. Periodic cleaning of database and consistency checks were regularly performed.
At scheduled intervals, a meeting of all participating centers was arranged to examine criticism, to discuss the results of analysis from cumulative data, and to organize the workload for the new semester. The directions to follow were decided by a Scientific Committee made up of Neuroradiologists and Neurologists. A technical coordinator center managed the database, contacted the centers, edited the newsletters and organized the meetings.
We performed a descriptive analysis of the study population, both for retrospective and prospective data. Only records gathered by centers with at least 80 % of completed data in the main efficacy and safety measures, including recanalization rates, intracranial hemorrhage, and 3-month functional outcome, were considered suitable for statistical analysis. This center-based selection was adopted to avoid selection bias.
Continuous variables were reported as median and interquartile range (iqr); categorical variables were reported as proportions. Statistical analysis was performed using SPSS software (version 20.0).
Results
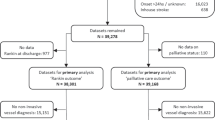
From January 1, 2008 to December 31, 2012, 1078 patients were treated in 32 centers (Appendix I). Seven centers, gathering 118 patients, were excluded from the analysis according to the center-based selection criteria, as exposed above (for a comparison of included and excluded patients see Appendix II). In the retrospective phase of the study, 16 centers participated in the registry; while 25 centers recorded patients in the prospective phase. Bridging and rescue treatments were performed, respectively, in 4 (25 %) and 10 (62 %) centers in the retrospective phase and in 9 (36 %) and 18 (64 %) centers in the prospective phase.
The study population included 960 patients, 321 retrospectively and 639 prospectively collected. Median age was 67.3 (iqr 54–75) years, male gender 57 %. Median NIHSS at baseline was 17 (iqr 13–22). Median time from symptoms onset to endovascular treatment was 4.5 (iqr 3.5–6.1) hours. More represented vascular risk factors were hypertension (49.8 %), dyslipidemia (21.9 %), smoking habit (19.9 %), and atrial fibrillation (19.9 %) (Table 1).
The most frequent site of occlusion was middle cerebral artery (46.9 %); carotid T-siphon and posterior circulation arteries were occluded in 21.4 and 21.8 % of cases, respectively. Direct access to the angiographic suite was adopted in 745 (80.4 %) patients, while 172 (19.6 %) patients were previously treated with intravenous thrombolysis. Of these latter, 14.2 % were treated at full doses and were sent to endovascular treatment as a rescue therapy, and 5.4 % received low doses of intravenous thrombolysis before endovascular treatment, according to the bridging protocol.
Concerning endovascular treatment modality, 165 (17.9 %) patients were treated with intra-arterial thrombolytic drugs (Urokinase or rt-PA), 531 (57.5 %) received a mechanical approach, including thrombectomy, thromboaspiration, and stent deployment, and 228 (24.7 %) patients received both a pharmacological and a mechanical treatment (Table 2).
In particular, UK and rt-PA were used in at least one step of treatment, respectively, in 27 and 39 % of the retrospective patients, and in 15 and 25 % of the prospective population. Thromboaspiration, performed either by means of manual suction thrombectomy or specific devices, was recorded in 21 % of the retrospective and in 18 % of the prospective patients.
Distal thrombectomy was performed by clot removal with all the commercially available specific devices including stentrievers (24 and 57 %, respectively, in the retrospective and the prospective population) or clot by-pass by means of angioplasty or stent deployment (15 % in the retrospective and 20 % in the prospective patients).
Angioplasty and/or stent deployment in extracranial arteries were also recorded in 8 % of the retrospective population and in 21 % of the prospective one.
Discussion
Our preliminary results show that baseline features of this cohort are in line with data in literature coming from large clinical series and recent clinical trials on endovascular stroke treatments [1–3, 7]. Avoiding narrow selection criteria for patients and being the recording not limited in time, this registry allows to collect data from a clinical practice scenario and to highlight real time changes in treatment modality.
Baseline features of the population are quite similar in the retrospective and the prospective cohorts. Middle cerebral artery is the most frequent site of occlusion, and thrombectomy the most employed technique, with a trend in time for a reduction in the use of pure pharmacological thrombolysis in favor of stentrievers. A trend towards an increase in time of bridging and rescue protocols is also shown.
A comparison between patients included and excluded from the analysis was performed, showing significant differences in baseline features between the two groups. In the excluded population, missing data in the main domains ranged from about 20 to 50 %. This could certainly lead to selection bias. We adopted center-based selection criteria to exclude all records gathered by centers which were not considered reliable, thus limiting the bias of selection possibly performed by single centers.
The strengths of our study are the large sample, the prospective nature of data collection, and the source of data from a current practice multicenter scenario. This allows to generalize the results and to translate findings into clinical practice, expanding and corroborating experimental evidence.
Limitations of this study include the lack of a control group and the voluntary participation of centers which prevents a central monitoring and a complete view of the national distribution of treating centers. Even though a control group is not provided for in a treatment registry, it could be possible to define a control population from other observational series matched by means of the propensity score.
This registry will provide a real-world view of safety and efficacy of endovascular procedures and of patient outcomes. It could improve our understanding on the appropriate selection of patients for endovascular treatment, and hopefully it will allow to develop suitable treatment algorithms for a specific kind of patient.
Moreover, a national-based registry could be valuable in assessing endovascular treatment utilization rates according to different regional distribution and availability, essential for policymaker in planning health service.
In the future, a wider analysis of the prospective data collected will provide useful information about everyday practice and trends in time for endovascular treatment of stroke. We believe data from our registry can address unsolved safety and efficacy concerns on endovascular treatment of ischemic stroke, providing a useful tool for the planning of new trials.
References
Broderick JP, Palesch YY, Demchuk AM et al (2013) Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 368:893–903
Ciccone A, Valvassori L, Nichelatti M et al (2013) Endovascular treatment for acute ischemic stroke. N Engl J Med 368:904–913
Kidwell CS, Jahan R, Gornbein J et al (2013) A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 368:914–923
Gliklich RE, Dreyer NA (eds) Registries for evaluating patient outcomes: a user’s guide, 2nd edn
Hacke W, Kaste M, Fieschi C et al (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet 352(9136):1245–1251
Higashida RT, Furlan AJ (2003) For the Technology Assessment Committees of the American Society of Interventional and Therapeutic Neuroradiology and the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34:e109–e137
del Zoppo GJ, Higashida RT, Furlan AJ et al (1998) PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT investigators. Prolyse in acute cerebral thromboembolism. Stroke 29:4–11
Acknowledgments
The project “Registro Nazionale Trattamento Ictus Acuto” (RFPS-2006-1-336562) was funded by grants from the Italian Ministry of Health within the framework of 2006 Finalized Research Programmes (D.Lgs.n.502/1992).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the Italian Registry of Endovascular Treatment in Acute Stroke.
Members of the Italian Registry of Endovascular Treatment in Acute Stroke are listed in “Appendix”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1
This appendix describes the registry organization and the participating centers.
Steering Committee
R. Gasparotti, Interventional Neuroradiology Unit, “Spedali Civili”, Brescia, Italy
D. Inzitari, Stroke Unit, University Hospital “Careggi”, Florence, Italy
S. Mangiafico, Interventional Neuroradiology Unit, University Hospital “Careggi”, Florence, Italy
D. Toni, Stroke Unit, University Hospital “Umberto I”, Rome, Italy
S. Vallone, Interventional Neuroradiology Unit, Nuovo Ospedale Civile “S.Agostino-Estense”, AUSL Modena, Italy
A. Zini, Stroke Unit, Nuovo Ospedale Civile “S.Agostino-Estense”, AUSL Modena, Italy
Scientific Committee
M. Bergui, Interventional Neuroradiology Unit, “San Giovanni Bosco” Hospital, Torino, Italy
F. Causin, Interventional Neuroradiology Unit, University Hospital, Padova, Italy
A. Ciccone, Department of Neurology and Stroke Unit, “Carlo Poma” Hospital, Mantua, Italy
P. Nencini, Stroke Unit, University Hospital “Careggi”, Florence, Italy
A. Saletti, Interventional Neuroradiology Unit, University Hospital “Arcispedale S. Anna”, Ferrara
F. Sallustio, Stroke Unit, UTN Policlinico Tor Vergata, Rome, Italy
R. Tassi, Stroke Unit, University Hospital “S. Maria delle Scotte”, Siena, Italy
F. Zappoli Thyrion, Diagnostic and Interventional Neuroradiology, “San Matteo” Hospital, Pavia, Italy
Data management and coordination unit
G. Pracucci, Department of Neurological and Psychiatric Sciences, Careggi University Hospital, Florence, Italy
V. Saia, Department of Neurological and Psychiatric Sciences, Careggi University Hospital, Florence, Italy
Participating centers: the following persons and institutions participates the registry (numbers of patients treated per center are in brackets)
-
1.
“Careggi” University Hospital, Florence (134). Interventional Neuroradiology Unit: S. Mangiafico S. Nappini, N. Limbucci. Stroke Unit: D. Inzitari, P. Nencini
-
2.
Nuovo Ospedale Civile “S.Agostino-Estense”, AUSL Modena (115). Neuroradiology Unit: P. Carpeggiani, S. Vallone. Stroke Unit -Neurology Clinic: A. Zini, G. Bigliardi, ML. Dell’Acqua
-
3.
Ospedale Civile “Mazzini”, Teramo (108). Vascular and Interventional Radiology Unit: V. Di Egidio, M. Fuschi, E. Puglielli. Neurology Unit: M. Assetta, D. Cerone
-
4.
Ospedale “Molinette”, Torino (82). Interventional Neuroradiology Unit: M. Bergui, G. Stura, D. Daniele. Stroke Unit: P.Cerrato, R. Palmiero
-
5.
Policlinico Tor Vergata, Rome (79). Interventional Neuroradiology Unit: R. Gandini, E. Pampana, A. Spinelli. Stroke Unit: P. Stanzione, F. Sallusti, G. Koch
-
6.
“Arcispedale S. Anna” University Hospital, Ferrara (56). Interventional Neuroradiology Unit: A. Saletti, E. Fainardi. Stroke Unit: A. De Vito, C. Azzini
-
7.
“S. Maria delle Scotte” University Hospital, Siena (45). Interventional Neuroradiology Unit: S. Bracco, DG. Romano, A. Cerase. Stroke Unit: G. Martini, R. Tassi
-
8.
University Hospital, Padova (43). Interventional Neuroradiology Unit: F. Causin, G. Cester. Stroke Unit -Neurology Clinic: C. Baracchini
-
9.
“Niguarda Cà Granda” Hospital, Milan (39). Interventional Neuroradiology Unit: L. Valvassori, M. Piano. Stroke Unit: E. Agostoni, C. Motto, A. Gatti
-
10.
“Spedali Civili”, Brescia (38) Interventional Neuroradiology Unit: R. Gasparotti, D. Mardighian. Stroke Unit: M. Magoni, A. Costa
-
11.
“AO Circolo e Fondazione Macchi” University Hospital, Varese (30). Neuroradiology Unit: A. Giorgianni, F. Baruzzi Stroke Unit: M.L. Delodovici, F. Carimati, G. Bono.
-
12.
“Ospedale dell’Angelo” Mestre (29). Neuroradiology Unit: E. Cagliari, N. Cavasin. Neurology Unit: R. Quatrale, A. Critelli
-
13.
“San Salvatore” University Hospital, L’Aquila (26). Neuroradiology Unit: M. Gallucci, AV. Giordano, S. Carducci. Neurology Unit: A. Carolei, S. Sacco, C. Tiseo
-
14.
“U. Parini” Regional Hospital, Aosta (20). Diagnostic and Interventional Radiology Unit: T. Meloni. M. Cristoferi, M. Natrella Neurology Unit: E. Bottacchi, G. Corso, P. Tosi
-
15.
“San Giovanni Bosco” Hospital, Torino (19). Interventional Neuroradiology Unit: G. Vaudano. Neurology Unit: R. Cavallo, E. Duc, G. Chianale
-
16.
“San Matteo” Hospital, Pavia (16). Diagnostic and Interventional Neuroradiology Unit: F.Zappoli, E. Lafe Emergency and Vascular Medicine: A. Martignoni. Stroke Unit: A. Cavallini, E. Candeloro, I. Canavero
-
17.
“Santa Corona” Hospital, Pietra Ligure (16). Neuroradiology Unit: R. Padolecchia, R. Schizzi, S. Calia. Neurology Unit: T. Tassinari, A. Sugo
-
18.
“A. Manzoni” Hospital, Lecco (14). Neuroradiology Unit: M. Longoni. Neurology and Stroke Unit: A. Salmaggi
-
19.
University Hospital, Verona (11). Neuroradiology Unit: P. Zampieri, DS Zimatore, A. Grazioli Stroke Unit: P. Bovi
-
20.
University Hospital, Pisa (11). Neuroradiology Unit: M. Puglioli, GA. Lazzarotti. Neurology Unit: G. Orlandi, A. Chiti, G. Gialdini
-
21.
“Umberto I” University Hospital, Rome (10). Interventional Neuroradiology Unit: G. Guidetti, S. Peschillo. Stroke Unit: D. Toni, A. Falcou, A. Anzini
-
22.
“Ospedale Maggiore”, Bologna (7). Interventional Neuroradiology Unit: L. Simonetti, A. Stafa, S. Isceri. Stroke Unit: G. Procaccianti, A. Zaniboni, A. Borghi
-
23.
University Hospital, Parma (5). Interventional Neuroradiology Unit: R. Menozzi, P. Piazza, S. Bruni. Stroke Unit: U. Scoditti, C. Zanferrari, P. Castellini
-
24.
“San Camillo-Forlanini” Hospital, Rome (4) . Diagnostic and Interventional Neuroradiology Unit: E. Cotroneo, F. Ricciardi, R. Gigli. Stroke Unit: C. Pozzessere, F.R. Pezzella, F. Corsi
-
25.
Cardarelli Hospital, Napoli (3). Neuroradiology Unit: M. Muto, GL. Guarnieri. Neurology Unit: V. Andreone
Appendix II
Comparison of the main features of patients included and excluded from the analysis, according to the completeness of data recorded in 1078 patients in the Endovascular Stroke Treatment Registry.
Patients included in the analysis N = 960 | Patients excluded from the analysis N = 118 | P | |
|---|---|---|---|
Age, year | 67.3 (54.0–74.8) | 66.7 (51.6–75.5) | 0.736 |
Missing | 0 (0 %) | 0 (0 %) | |
Gender M | 547 (57.0) | 71 (60.2) | 0.508 |
Missing | 0 (0 %) | 0 (0 %) | |
Baseline NIHSS score | 17 (13–22) | 18 (14–21) | 0.881 |
Missing | 96 (10.0 %) | 57 (48.3 %) | |
Time to admission* | 2.5 (1.2–4.0) | 1.5 (1.0–5.9) | 0.190 |
Missing | 74 (7.7 %) | 42 (35.6 %) | |
Time to groin puncture^ | 4.5 (3.5–6.1) | 11.5 (4.2–16.2) | <0.001 |
Missing | 39 (4.1 %) | 29 (24.6 %) | |
Site of occlusion | |||
Missing | 34 (3.5 %) | 26 (22.0 %) | |
Carotid T-syphon§ | 198 (21.4) | 8 (8.7) | <0.001 |
Middle Cerebral Artery | 434 (46.9) | 49 (53.3) | |
Vertebral-Basilar Arteries | 202 (21.8) | 14 (15.2) | |
Other sites^^ | 92 (9.9) | 21 (22.8) | |
IV/IA Treatment | |||
Missing | 33 (3.4 %) | 28 (23.7 %) | |
IA treatment | 745 (80.4) | 88 (97.8) | <0.001 |
Bridging# | 50 (5.4) | 2 (2.2) | |
Rescue## | 132 (14.2) | 0 (0.0) | |
IA Treatment Modality | |||
Missing | 36 (3.7 %) | 27 (22.9 %) | |
IA pharmacological thrombolysis | 165 (17.9) | 19 (20.9) | 0.303 |
Thrombectomy | 531 (57.5) | 56 (61.5) | |
Pharmacological thrombolysis + thrombectomy | 228 (24.7) | 16 (17.6) | |
Rights and permissions
About this article
Cite this article
Mangiafico, S., Pracucci, G., Saia, V. et al. The Italian Registry of Endovascular Treatment in Acute Stroke: rationale, design and baseline features of patients. Neurol Sci 36, 985–993 (2015). https://doi.org/10.1007/s10072-014-2053-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-2053-5