Abstract
microRNAs (miRNAs) are a class of small non-coding RNAs, approximately 21–25 nucleotides in length. Recently, some researchers have demonstrated that plasma miRNAs are sensitive and specific biomarkers of various cancers. Using quantitative real-time PCR, the expression levels of miR-454-3p were compared between pre-operative plasmas from 70 glioma patients and 70 healthy controls, and between these pre-operative and post-operative plasmas. Kaplan–Meier analysis was used to evaluate the association of miR-454-3p with prognosis of glioma patients. The expression levels of miR-454-3p in plasma in glioma patients were significantly higher than that from healthy controls. The expression levels of miR-454-3p were higher in high grade gliomas than in low grade gliomas. The AUC of the expression of miR-454-3p in plasma for glioma diagnosis was 0.9063. The expression levels of miR-454-3p in the post-operative plasmas were significantly downregulated when compared to the pre-operative plasmas. Moreover, the prognosis of glioma with high miR-454-3p expression was significantly worse compared with that of glioma with low miR-454-3p expression. Our results suggested that plasma miR-454-3p could be a novel potential diagnostic biomarker for glioma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common primary malignant brain tumors, which comprise approximately one-third of intrinsic neoplasms of the central nervous system in both adults and children [1]. It is a type of aggressive tumor with a tendency to invade the surrounding brain tissue. The patients diagnosed with glioblastoma multiforme (GBM, a grade IV glioma) remain poor prognosis despite implementation of intensive therapeutic strategies and clinical efforts [2]. To date, the diagnosis of glioma before clinical treatment is mainly by computer tomography and nuclear magnetic resonance imaging. However, they are expensive and difficult to spread. Therefore, it is an urgent need to find new approaches to early diagnose glioma and monitor disease progress.
MicroRNAs (miRNAs) are a class of highly conserved, single-stranded, small non-coding RNA molecules, which are known as endogenous regulators of post-transcriptional gene expression regulating expression through translational repression and messenger RNA cleavage [3, 4]. Through this mechanism of post-transcriptional gene regulation, miRNAs play crucial regulatory roles in a wide range of biological processes, including cellular proliferation and differentiation, migration, apoptosis, development and metabolism, and their altered expression contributes to the pathogenesis of many human diseases, including cancers [5–7]. For example, Hou et al. [8] found that miR-205 was a potential prognostic indicator for human glioma. Interestingly, more and more researchers have found that circulating miRNAs in plasma or serum could be used as potential biomarkers for detection, identification, and classification of cancers and other diseases [9, 10]. Roth et al. [11] demonstrated that specific miRNAs in peripheral blood may be suitable biomarkers for glioblastoma. Wang et al. [12] found that miR-21-5p, miR-128-3p and miR-342-3p were significantly altered in the plasma of glioma patients and were predictive for the diagnosis of glioma. Ilhan-Mutlu et al. reported that plasma miR-21-5p may be a useful biomarker to be associated with glioblastoma development in individual cases [13, 14]. However, the expression pattern of miR-454-3p in glioma plasma and its association with clinicopathologic characteristics have not been reported yet. The primary aim of the study was to investigate the potential of miR-454-3p in plasma as a diagnostic biomarker of glioma patients, and the relationship of miR-454-3p with glioma clinicopathologic characteristics and prognosis of patients.
Materials and methods
Clinical samples
The study was approved by Research Ethics Committee of The Second Affiliated Hospital of Soochow University and the Research Ethics Committee of The Third Affiliated Hospital of Soochow University. All clinical samples described here were gained from patients who had given informed consent and stored in the hospital database.
Plasma samples were collected from 70 patients with confirmed glioma before and 14 days after surgery at Department of Neurosurgery, The Second Affiliated Hospital of Soochow University from September 2010 to December 2013. Plasmas from 70 healthy individuals were collected as normal controls. The detailed characteristics of these patients are shown in Table 1. In the validation set, plasma samples were collected from 30 glioma patients before surgery and from 30 healthy individuals at The Third Affiliated Hospital of Soochow University from June 2012 to June 2014. EDTA blood samples were collected from cases and control individuals and processed for plasma within 2 h of collection. To avoid contamination with epithelial cells from the initial skin puncture the first blood tube collected during phlebotomy was not processed for plasma. The blood samples were obtained and centrifuged for 10 min at 1,500g within 2 h after collection, and the supernatant was removed to RNase-free tubes and further centrifuged for 10 min at 12,000g and 4 °C to remove cells and debris. Plasma was stored at −80 °C until further processing.
miRNAs isolation and quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was extracted from 400 μl plasma with the miRNeasy Mini Kit (Qiagen), in accordance with the manufacturer’s instructions. The RNA quality and concentration were assessed using a NanoDrop ND1000 Spectrophotometer (ThermoFisher Scientific, Waltham, Mass).
Plasma RNA containing miRNA was polyadenylated by poly(A) polymerase and reverse transcribed to cDNA using TaqMan MicroRNA Reserve Transcription Kit and TaqMan MicroRNA Primer (Applied Biosystem, Austin, TX) according to the manufacturer’s instructions. Real-time qPCR was performed using TapMan MicroRNA Assays, TaqMan 2× Universal PCR Master Mix and No AmpErase UNG (Applied Biosystem) in ABI PRISM 7500 Real-time PCR system (Applied Biosystems). The amplification profile was: 95 °C 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Due to the lack of a consensus housekeeping miRNA for qRT-PCR analysis of plasma miRNA, we selected a normalization method utilizing the comparison of the miRNA concentration to the volume, as was described in previous report [15]. After the reactions were completed, the threshold cycle (Ct) values were determined using the default threshold settings. Each sample was run in triplicates for analysis. The relative amount of miR-454-3p was calculated based on the equation 2−ΔCt, in which ΔCt = Ct miR-454-3p − Ct cel-miR-39.
Statistical analysis
The significance of plasma miRNA level was determined by Mann–Whitney test, Wilcoxon test and χ 2 test where appropriate using the SPSS software (version 13.0) and Graphpad Prism (version 5.0). Receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) were established for discriminating patients with glioma. Cut-off value of the expression of miR-454-3p was set by Youden index from ROC curves, which is determined at the top left point on the ROC curve when the difference is maximal between sensitivity and 1-specificity. p values of two sided less than 0.05 was considered statistically significant.
Results
Patient characteristics
Clinicopathological characteristics of 70 patients with glioma and 70 healthy controls were summarized in Table 1. There were no significant differences of gender and age between glioma patients and healthy controls.
The expression levels of miR-454-3p in plasma
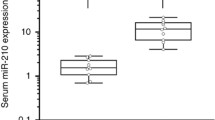
The expression levels of miR-454-3p in plasma in 70 glioma patients were significantly higher than that from healthy controls (p < 0.0001) (Fig. 1a). Furthermore, to determine whether miR-454-3p could specifically and sensitively discriminate glioma patients from healthy controls, ROC curves were constructed. The ROC curves analysis showed that at the optimal cut-off, plasma miR-454-3p had a 99.05 % sensitivity and a 82.86 % specificity and the area under the ROC curve (AUC) was 0.9063 [95 % confidence interval (CI): 0.8487–0.9639)] (Fig. 1b). Furthermore, the relationship between the plasma levels of miR-454-3p and histopathological grade of glioma was also studied. The plasma levels of miR-454-3p were stratified using three types of clinicopathological parameters (gender, age and WHO grade). miR-454-3p was not differentially expressed when the clinical samples were stratified by age or gender. However, the plasma levels of miR-454-3p were significantly higher in high grade (WHO grade III and IV) than in low grade (WHO grade I and II) samples (p = 0.013, Fig. 1c). Furthermore, we tested another 60 plasma samples (30 gliomas and 30 controls) from the Third Affiliated Hospital of Soochow University to confirm the expression of miR-454-3p in glioma plasma. As shown in Supplementary Fig. 1, the expression levels of miR-454-3p in plasma in 30 glioma patients were significantly higher than that from healthy controls (p < 0.0001).
The expression levels of miR-454-3p in plasma from healthy controls and glioma patients. a The expression levels of plasma miR-454-3p in glioma patients and in healthy controls. b The ROC curve reflecting the ability of the plasma levels of miR-454-3p to differentiate the glioma patients from the controls. c The expression levels of plasma miR-454-3p in low grade and high grade glioma patients
The change of miR-454-3p in plasma samples of glioma patients after operation
Subsequently, the plasma levels of miR-454-3p were analyzed in paired pre- and post-operative plasma samples from the patients who underwent surgical removal of the tumors. It was found that the expression levels of miR-454-3p in the post-operative plasmas were significantly downregulated when compared to the pre-operative plasmas (p = 0.0015, Fig. 2).
Correlation between the expression levels of miR-454-3p and the survival of glioma patients
We next investigated the correlation between the expression levels of miR-454-3p and the survival outcome using the prospective follow-up data collected from the 70 glioma patients. The expression levels of miR-454-3p were first stratified by the median value; then, the survival outcome of patients with high miR-454-3p expression levels (≥median) was compared with those exhibiting low miR-454-3p expression levels (<median), as determined by the Kaplan–Meier survival analysis. We observed a poorer, marginally significant survival rate in glioma patients who expressed high levels of miR-454-3p (p = 0.040, Fig. 3).
Discussion
Gliomas are the most common primary tumors of the central nervous system. Despite multimodal treatment options, the overall prognosis for most patients is poor, in particular in the case of GBM. Currently available combined therapies offer only limited benefits for patients with glioma. It is imperative to develop novel therapeutic approaches for the diagnosis and prognosis of glioma. In the present study, our results showed that miR-454-3p was upregulated in plasma samples of human glioma before surgery compared to healthy controls. ROC analysis demonstrated the high sensitivity and specificity of miR-454-3p for glioma diagnosis. The expression levels of miR-454-3p were higher in high grade than in low grade gliomas which suggested that the upregulation of miR-454-3p in glioma plasma was associated with advanced clinical stages of glioma. Furthermore, the expression levels of miR-454-3p were significantly downregulated after surgery. Finally, high expression levels of miR-454-3p were correlated with poor prognosis indicating the prognostic value of miR-454-3p for glioma patients. Thus, these findings suggested that miR-454-3p in plasma could be a potential circulating diagnostic and prognostic marker for glioma.
miRNAs are known to play crucial roles as oncogenes or tumor suppressors, and their deregulation is involved in multiple processes including cell proliferation, apoptosis, cell-cycle regulation and invasion in various diseases [3, 6]. miR-454-3p is differentially expressed in different diseases. miR-454-3p was downregulated in esophageal cancer [16]. miR-454-3p was increased in cirrhotic livers and had a significant negative correlation with hepatic CYP3A activity [17]. Plasma miR-454-3p was upregulated in myotonic dystrophy type 1 patients [18]. Serum miR-454-3p is downregulated in diastolic dysfunction [19]. In this study, the upregulation of plasma miR-454-3p level was found in the glioma. It indicates that upregulation of miR-454-3p level in plasma is an early affair and may have some roles of carcinogenesis for glioma. However, the mechanism of miR-454-3p in glioma carcinogenesis still needs to be determined. It has been reported that miR-454-3p could inhibit the activation of hepatic stellate cells by directly targeting Smad4 [20]. Smad proteins are phosphorylated and activated by transmembrane serine–threonine receptor kinases in response to TGF-beta signaling and are subject to complex regulation by post-translational modifications. Mutations or deletions in these genes have been shown to be involved in many cancers. There have been no direct reports about the role of miR-454-3p in glioma progression, thus its molecular and biological function, pathways to the target genes, and regulation mechanism need further studies.
The discovery that circulating miRNAs can serve as potential cancer biomarkers could overcome the problem of collecting tissue samples through invasive procedures such as biopsy or surgery. Serum samples are easily acquired in a relatively non-invasive manner, and isolated miRNAs are readily detected by qRT-PCR. Therefore, this technique would allow the comprehensive analysis of diseases in a less invasive manner and in an early stage. However, the mechanism of origin of extracellular miRNAs remains to be fully elucidated. It is widely believed that miRNAs released from damaged cells or circulating cells lead to increased plasma miRNA expressions [21]. The exact reason for plasma miR-454-3p levels increase in glioma is not clear. We suspect that (1) blood brain barrier (BBB) may be partly destroyed in patients with glioma; (2) exosomes or some other complexes may carry miR-454-3p out of BBB by unknown mechanisms. It has been shown that cell to cell communication can be mediated by exosomes containing miRNAs [22]. Recent data showed that miRNAs in extracellular vesicles facilitate communication between tumor cells and endothelial cells, and endothelial cells and smooth muscle cells, suggesting that cancer-related circulating miRNAs may be functional in the similar pattern [23].
In summary, our results suggest that miR-454-3p levels in plasma may serve as a potential non-invasive biomarker for the diagnosis and prognosis of glioma.
References
Siegel R, Naishadham D (2013) Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30
Davis FG, McCarthy BJ (2001) Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther 1(3):395–401
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6(4):259–269
Cech TR, Steitz JA (2014) The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157(1):77–94
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11):857–866
Palanichamy JK, Rao DS (2014) miRNA dysregulation in cancer: towards a mechanistic understanding. Front Genet 5:54
Hou SX, Ding BJ, Li HZ, Wang L, Xia F, Du F, Liu LJ, Liu YH, Liu XD, Jia JF, Li L, Wu ZL, Zhao G, Zhang ZG, Deng YC (2013) Identification of microRNA-205 as a potential prognostic indicator for human glioma. J Clin Neurosci 20(7):933–937
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18(10):997–1006
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105(30):10513–10518
Roth P, Wischhusen J, Happold C, Chandran PA, Hofer S, Eisele G, Weller M, Keller A (2011) A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem 118(3):449–457
Wang Q, Li P, Li A, Jiang W, Wang H, Wang J, Xie K (2012) Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res 31:97
Ilhan-Mutlu A, Wagner L, Wohrer A, Furtner J, Widhalm G, Marosi C, Preusser M (2012) Plasma MicroRNA-21 concentration may be a useful biomarker in glioblastoma patients. Cancer Invest 30(8):615–621
Ilhan-Mutlu A, Wagner L, Wohrer A, Jungwirth S, Marosi C, Fischer P, Preusser M (2012) Blood alterations preceding clinical manifestation of glioblastoma. Cancer Invest 30(9):625–629
Shen W, Song M, Liu J, Qiu G, Li T, Hu Y, Liu H (2014) MiR-26a promotes ovarian cancer proliferation and tumorigenesis. PLoS One 9(1):e86871
Liu SG, Qin XG, Zhao BS, Qi B, Yao WJ, Wang TY, Li HC, Wu XN (2013) Differential expression of miRNAs in esophageal cancer tissue. Oncol Lett 5(5):1639–1642
Vuppalanchi R, Liang T, Goswami CP, Nalamasu R, Li L, Jones D, Wei R, Liu W, Sarasani V, Janga SC, Chalasani N (2013) Relationship between differential hepatic microRNA expression and decreased hepatic cytochrome P450 3A activity in cirrhosis. PLoS One 8(9):e74471
Perfetti A, Greco S, Bugiardini E, Cardani R, Gaia P, Gaetano C, Meola G, Martelli F (2014) Plasma microRNAs as biomarkers for myotonic dystrophy type 1. Neuromuscul Disord 24(6):509–515
Nair N, Kumar S, Gongora E, Gupta S (2013) Circulating miRNA as novel markers for diastolic dysfunction. Mol Cell Biochem 376(1–2):33–40
Zhu D, He X, Duan Y, Chen J, Wang J, Sun X, Qian H, Feng J, Sun W, Xu F, Zhang L (2014) Expression of microRNA-454 in TGF-beta1-stimulated hepatic stellate cells and in mouse livers infected with Schistosoma japonicum. Parasit Vectors 7:148
Zen K, Zhang CY (2012) Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev 32(2):326–348
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9(6):654–659
Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S (2012) Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 14(3):249–256
Acknowledgments
This work was supported by National Natural Science Foundation of China Projects 31071046 and 81302197, Changzhou Science Development Project (CS20102010) and Changzhou Health Bureau Key Project (ZD201007).
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
N. Shao and L. Wang contributed equally in this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, N., Wang, L., Xue, L. et al. Plasma miR-454-3p as a potential prognostic indicator in human glioma. Neurol Sci 36, 309–313 (2015). https://doi.org/10.1007/s10072-014-1938-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1938-7