Abstract
The aim of the present study was to investigate the clinical significance of microRNA-124 (miR-124) expression in glioma. The expression levels of miR-124 were measured using quantitative real-time polymerase chain reaction (qRT-PCR) analysis. The correlation between the miR-124 levels and the clinicopathological factors of the glioma patients was analyzed. The survival curves were calculated by the Kaplan–Meier method. The influence of each variable on survival was examined by the Cox multivariate regression analysis. Compared with nonneoplastic brain tissues, the expression level of miR-124 was significantly decreased in glioma tissues (1.27 ± 0.55 versus 6.91 ± 1.06, P < 0.0001). The expression level of miR-124 was positively correlated with grade (P = 0.003) and Karnofsky performance status (KPS) score (P = 0.008). A significant difference was found that glioma patients with low miR-124 expression level had distinctly shorter OS (P = 0.001) and PFS (P = 0.002) than patients with high miR-124 expression level. Furthermore, we found that low miR-124 expression (OS P = 0.009; PFS P = 0.002) and advanced histologic grade (OS P = 0.005; PFS P = 0.001) were independent prognostic parameters indicating poor prognosis for glioma patients. Our results showed that the decreased expression of miR-124 may be associated with malignant tumor progression and poor prognosis in patients with gliomas, suggesting that miR-124 may be a novel and valuable signature for predicting the clinical outcome of patients with gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioma accounts for most in human malignant central nervous system neoplasms [1]. Approximately 20,000 new cases of glioma are diagnosed in the United States every year [2]. World Health Organization (WHO) divides gliomas into four grades: pilocytic astrocytoma (WHO grade I), diffuse astrocytoma (WHO grade II), anaplastic astrocytoma (WHO grade III), and glioblastoma (WHO grade IV) [3]. Glioma is characterized by a rapid infiltrative growth pattern making complete surgical resection impossible. The median survival from glioblastoma multiforme, the most aggressive type of malignant glioma, is only 12–14 months, even when treated with surgery, chemotherapy, and/or radiotherapy [4]. Early detection and effective treatment strategies for glioma patients are vital for improving clinical outcomes, and developing biomarkers for this purpose has long been an aim of research. The information that biomarkers provide about cancer can be used to predict important factors such as prognosis and response to therapy, as well as to improve diagnosis and to assist earlier detection.
MicroRNAs (miRNAs) are small non-coding RNA molecules that are normally around 22 nucleotides in length. MiRNA function to regulate cell behavior and modulate gene expression at the post-transcriptional level, by binding to mRNA and suppressing translation [5, 6]. Previously, as one of the most abundantly expressed miRNAs in the central nervous system, miR-124 has been shown to play a significant role in the pathogenesis of various cancers [7–13]. To date, several lines of evidence have shown that miR-124 may be a novel prognostic factor and therapeutic target for some types of cancers, such as non-small cell lung cancer (NSCLC) [12], hepatocellular carcinoma (HCC) [8], oral squamous cell carcinoma (OSCC) [10], and colorectal cancer (CRC) [7]. However, the role of miR-124 in glioma and its association with prognosis have not been reported. The aim of the present study was to investigate the clinical significance of miR-124 expression in glioma.
Materials and methods
Patients and tissue samples
The present study was approved by the Research Ethics Committee of Qilu Hospital of Shandong University. Written informed consent was obtained from all the patients. All specimens were handled and made anonymous according to the ethical and legal standards. A total of 137 pairs of human gliomas and matched nonneoplastic brain tissues obtained from 137 patients with primary glioma were collected from Department of Neurosurgery, Qilu Hospital of Shandong University between 2006 and 2012. Samples resected during the operation were divided into two parts. One part was frozen and stored in liquid nitrogen until RNA isolation, the other one was fixed in formalin for pathologic diagnosis by two pathologists according to the 2007 WHO classification. Any different conclusions were resolved by careful study and discussion. The patients were 77 male and 60 female, with a median age of 45 years (range 21–79 years). According to WHO criteria, 66 of the 137 gliomas were classified as low-grade [32 pilocytic astrocytomas (WHO I) and 34 diffuse astrocytomas (WHO II)], and 71 were classified as high-grade gliomas [32 anaplasia astrocytomas (WHO III), and 39 primary glioblastomas (WHO IV)]. None of the patients had received chemotherapy or radiotherapy prior to surgery. The clinicopathologic features of all the patients were summarized in Table 1. All patients were under a close follow-up observation for disease recurrence at no less than 3-month intervals during the first two postoperative years, and no less than every 6 months thereafter. Overall survival (OS) time was calculated from the date of the initial surgery to death. Progression-free survival (PFS) time was calculated from the date of the initial surgery until the first evidence of local, regional, or distant tumor progression of disease.
Real-time quantitative RT-PCR for miRNA
MiR-124 expression in human gliomas and nonneoplastic brain tissues was measured by reverse transcription and real-time PCR (RT-PCR). Total RNA was isolated from frozen samples using Trizol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. The TaqMan microRNA assay and TaqMan universal PCR master mix were used to detect the expression of miR-124, and the U6 gene was used as an internal control to normalize variances. The cycle threshold (C T) value was calculated. The \(2^{{ - \Delta C_{\text{T}} }}\) (ΔC T = C TmiR124 − CTU6 RNA) method was used to quantify the relative amount of miR-124. Each sample was examined in triplicate and the raw data were presented as the relative quantity of target miRNA, normalized with respect to U6. RT-PCR primers: miR-124: F: 5′-CTAGTCTAGAGTCGCTGTTATCTCATTGTCTG-3′; R: 5′-CGCGGATCCTCTGCTTCTGTCACAGAATC-3′; U6: F: 5′-GCGCGTCGTGAAGCGTTC-3′; R: 5′-GTGCAGGGTCCGAGGT-3′.
Statistical analysis
All computations were carried out using SPSS 18.0 for Windows (SPSS Inc., IL, USA). The expression of miR-124 was assessed for associations with clinicopathological characteristics using Chi-square test. The survival curves were calculated by the Kaplan–Meier method and the difference by the log-rank test. The influence of each variable on survival was examined by the Cox multivariate regression analysis. All P values cited were two sided, and P values <0.05 were judged as statistically significant.
Results
Decreased expression of miR-124 in human glioma tissues
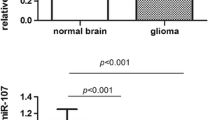
The expression levels of miR-124 in all glioma tissues with WHO grade I–IV and nonneoplastic brain tissues are shown in Fig. 1. Compared with nonneoplastic brain tissues, the expression level of miR-124 was significantly decreased in glioma tissues (1.27 ± 0.55 versus 6.91 ± 1.06, P < 0.0001). The results showed that the highest expression of miR-124 was achieved in nonneoplastic brain tissues, while grade IV glioma tissue displayed the lowest expression level. Interestingly, the expression levels of miR-124 in glioma tissues with four WHO grades were all significantly lower than that in nonneoplastic brain tissues (shown in Fig. 1).
Association of miR-124 expression with clinicopathological parameters of glioma patients
Table 1 summarizes the association between miR-124 expression and clinicopathological parameters in gliomas. A significant relationship was observed between miR-124 expression and the histopathologic grade. We found that the glioma tissues from high-grade tumors (grade III and IV) had much lower miR-124 expression level than glioma tissues from low-grade tumors (grade I and II) (P = 0.003). Moreover, the expression level of miR-124 was positively correlated with KPS scores of glioma tissues (P = 0.008). No statistically significant association of miR-124 expression with age at diagnosis, gender of patients, extent of resection, and tumor size was found (all P > 0.05, shown in Table 1).
Low-expression level of miR-124 predicts poor prognosis in glioma patients
To determine the prognostic value of miR-124 expression in human glioma, clinical follow-up was available for all patients. Kaplan–Meier method and log-rank test were used to evaluate the differences of OS and PFS between low-expression group and high-expression group. A significant difference was found that glioma patients with low miR-124 expression level had distinctly shorter OS (P = 0.001) and PFS (P = 0.002) than patients with high miR-124 expression level (shown in Figs. 2, 3).
Univariate and multivariate analyses were utilized to evaluate whether the miR-124 expression level and various clinicopathological features were independent prognostic parameters of patient outcomes. The results of analysis are shown in Tables 2 and 3. We could find that low miR-124 expression (OS P = 0.009; PFS P = 0.002) and advanced histologic grade (OS P = 0.005; PFS P = 0.001) were independent prognostic parameters indicating poor prognosis for glioma patients.
Discussion
Despite the recent advances in tumor diagnosis and treatment, including surgery, radiotherapy and chemotherapy, the median survival time is only 1 year and few patients survive for 2 years. The 5-year survival rate of low-grade glioma is 30–70 %, while the median survival time of the most aggressive type, GBM, which grows and infiltrates rapidly, is from 9 to 12 months [14]. Several clinicopathologic features have been used as prognostic factors for gliomas, such as histopathologic grades and KPS score. Unfortunately, these factors cannot estimate the prognosis of glioma patients exactly because of patients’ heterogeneous [15]. Accumulating evidence reveals that a number of biological and molecular factors are involved in the development, progression, metastasis, and drug resistance of gliomas. However, few molecular signatures have been validated and widely accepted as prognostic indicators in clinical practice. Since more precise prognostic predictors and more effective therapies for gliomas are required, it is extremely necessary to identify novel molecular signatures that can predict the clinical outcome and response to treatment of this disease with reliable clinical significance.
In the current study, we examined the miR-124 expression in glioma tissues at various WHO grades. We found that the tissues from high-grade glioma had much lower miR-124 expression level than low-grade glioma and nonneoplastic brain tissues. Kaplan–Meier analysis showed that glioma patients with low miR-124 expression level had distinctly shorter OS and PFS. Furthermore, univariate and multivariate analyses showed that the miR-124 expression level was an independent prognostic parameter of patient outcomes. All the results suggested that miR-124 was befitting to predict prognosis of glioma patients after surgery.
The distinct miRNA expression patterns in different cancer types have been identified by profiling approaches. The functional discovery of miRNAs may enable to offer insight into regulation of gene expression and complexity of carcinogenesis. Interestingly, the influence of miRNAs may be dependent on cancer type. Some miRNAs act as oncogenes, by contributing to the transformed phenotype when expressed at high levels in cancers. These oncogenic miRNAs may function by suppressing tumor suppressor genes. In contrast, some miRNAs act as tumor suppressors by allowing the expression of oncogenes, and are weakly expressed or absent in tumors [16]. As a potential tumor suppressor gene, miR-124 has been shown to regulate growth, proliferation, apoptosis, migration, and invasion of tumor cells in certain cancers [10, 11, 17–19]. The tumor-specific hypermethylation-mediated silencing of miR-124 was a relatively frequent molecular event in gastric, cervical, and breast cancer [11, 17, 20]. Previously, Shi and colleagues found that expression levels of miR-124 were greatly downregulated in glioma specimens. Related Ras viral oncogene homolog (R-Ras) and neuroblastoma Ras viral oncogene homolog (N-Ras) were identified as direct targets of miR-124. MiR-124 inhibited glioma cell growth, invasion, angiogenesis, and tumor growth and increased chemosensitivity to temozolomide treatment by negatively regulating the Ras family and its downstream signaling pathways: phosphatidylinositol-3 kinase/Akt and Raf/extracellular signal-regulated kinase 1/2. Furthermore, overexpression of R-Ras rescued the inhibitory effects of miR-124. Meanwhile, overexpression of R-Ras and N-Ras restored miR-124-inhibited vascular endothelial growth factor (VEGF) transcription activation. In clinical glioma specimens, protein levels of R-Ras and N-Ras were upregulated and inversely correlated with miR-124 expression levels. Taken together, these results revealed that miR-124 levels in tumor tissues were associated with glioma occurrence, angiogenesis, and chemoresistance [21]. However, the role of miR-124 in glioma and the association with prognosis have not been reported previously. To the best of our knowledge, this is the first study to investigate the relationship between miR-124 expression level and the prognosis of glioma patients. We showed that miR-124 was a significant predictor of OS and PFS and an independent prognostic factor for glioma. The similar conclusion was reported by Zheng et al. [9] that low expression level of miR-124 was an independent prognostic factor for poor outcome in HCC patients. Wang and colleagues also reported that the downregulation expression of miR-124 was an independent prognostic factor in patients with CRC [7]. Therefore, our results were in line with previous studies.
Conclusions
In summary, our results showed that the decreased expression of miR-124 may be associated with malignant tumor progression and poor prognosis in patients with gliomas, suggesting that miR-124 may be a novel and valuable signature for predicting the clinical outcome of patients with gliomas. A more in-depth and larger scale study remains to identify the role of miR-124 in glioma.
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Davis FG, McCarthy BJ (2001) Current epidemiological trends and surveillance issues in brain tumors. Expert Rev Anticancer Ther 1:395–401
Nakazato Y (2008) The 4th Edition of WHO Classification of Tumours of the Central Nervous System published in 2007. No shinkei geka. Neurol Surg 36:473–491
Stupp R, Mason WP, Van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for, R, Treatment of Cancer Brain, T, Radiotherapy, G, National Cancer Institute of Canada Clinical Trials, G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Rao P, Benito E, Fischer A (2013) MicroRNAs as biomarkers for CNS disease. Front Mol Neurosci 6:39
Baraniskin A, Kuhnhenn J, Schlegel U, Maghnouj A, Zollner H, Schmiegel W, Hahn S, Schroers R (2012) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14:29–33
Wang MJ, Li Y, Wang R, Wang C, Yu YY, Yang L, Zhang Y, Zhou B, Zhou ZG, Sun XF (2013) Downregulation of microRNA-124 is an independent prognostic factor in patients with colorectal cancer. Int J Colorectal Dis 28:183–189
Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, Karin M, Struhl K, Hadzopoulou-Cladaras M, Iliopoulos D (2011) An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 147:1233–1247
Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, Kung HF, Xie D (2012) The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 61:278–289
Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW (2011) MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1. FEBS Lett 585:187–192
Wilting SM, van Boerdonk RA, Henken FE, Meijer CJ, Diosdado B, Meijer GA, le Sage C, Agami R, Snijders PJ, Steenbergen RD (2010) Methylation-mediated silencing and tumour suppressive function of HSA-miR-124 in cervical cancer. Mol Cancer 9:167
Patnaik SK, Kannisto E, Knudsen S, Yendamuri S (2010) Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res 70:36–45
Pierson J, Hostager B, Fan R, Vibhakar R (2008) Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma. J Neurooncol 90:1–7
Zhang X, Yang H, Gong B, Jiang C, Yang L (2012) Combined gene expression and protein interaction analysis of dynamic modularity in glioma prognosis. J Neurooncol 107:281–288
Chu SH, Ma YB, Feng DF, Zhang H, Zhu ZA, Li ZQ, Jiang PC (2012) Correlation of low SLC22A18 expression with poor prognosis in patients with glioma. J Clin Neurosci Off J Neurosurg Soc Australas 19:95–98
Chuang JC, Jones PA (2007) Epigenetics and microRNAs. Pediatr Res 61:24R–29R
Lv XB, Jiao Y, Qing Y, Hu H, Cui X, Lin T, Song E, Yu F (2011) miR-124 suppresses multiple steps of breast cancer metastasis by targeting a cohort of pro-metastatic genes in vitro. Chin J Cancer 30:821–830
Ponomarev ED, Veremeyko T, Barteneva NS (2011) Visualization and quantitation of the expression of microRNAs and their target genes in neuroblastoma single cells using imaging cytometry. BMC Res Notes 4:517
Furuta M, Kozaki KI, Tanaka S, Arii S, Imoto I, Inazawa J (2010) miR-124 and miR-203 are epigenetically silenced tumor-suppressive microRNAs in hepatocellular carcinoma. Carcinogenesis 31:766–776
Ando T, Yoshida T, Enomoto S, Asada K, Tatematsu M, Ichinose M, Sugiyama T, Ushijima T (2009) DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: its possible involvement in the formation of epigenetic field defect. Int J Cancer 124:2367–2374
Shi Z, Chen Q, Li C, Wang L, Qian X, Jiang C, Liu X, Wang X, Li H, Kang C, Jiang T, Liu LZ, You Y, Liu N, Jiang BH (2014) MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro Oncol. doi:10.1093/neuonc/nou084
Conflict of interest
There are no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, T., Wang, Xy., Li, C. et al. Downregulation of microRNA-124 predicts poor prognosis in glioma patients. Neurol Sci 36, 131–135 (2015). https://doi.org/10.1007/s10072-014-1895-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1895-1