Abstract
Angiogenesis has recently become a major target for the development of new antineoplastic drugs. The most serious adverse events linked to angiogenesis inhibitors are venous or arterial thromboembolism and haemorrhage. Thus, there is need to define with more certainty the impact of these new drugs in terms of adverse effects in neurological patients. The aim of the study is to assess the risk of venous thromboembolism (VTE) and bleeding in patients with malignant gliomas treated with bevacizumab with or without concomitant anticoagulant therapy. A review of published literature was performed in Medline, from which 476 records were identified. A total of 27 full-text articles, including retrospective analyses, retrospective reviews, and open label trials, were assessed for eligibility. The investigated drugs included bevacizumab alone, bevacizumab plus chemotherapy with/without concomitant radiation therapy; only two articles dealt with bevacizumab in association with anticoagulant treatment. A total of 2,208 patients with malignant gliomas, were identified and included in the analysis. From data it appears that patients receiving bevacizumab had a major risk of developing VTE that increased when bevacizumab is associated with radio-chemotherapy (4.27 vs 7.46 %). Regarding bleeding, data showed that patients treated with anticoagulant had a significantly increased risk of severe central nervous system (CNS) bleeding compared to patients not receiving anticoagulant therapy (0.6 vs 8.2 %). The use of bevacizumab combined with chemo-radiotherapy seems to be associated with a higher risk for VTE compared to patients receiving antiangiogenic therapy alone. The associated use of anticoagulants and bevacizumab far increases the risk of developing CNS and non-CNS bleeding higher than grade 3, compared to patients receiving bevacizumab alone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis of patients with glioblastoma (GBM) remains poor despite considerable therapeutic progress in neuro-oncology: the overall median survival (m-OS) of patients treated with current standard chemo-radiotherapy regimen is approximately 15 months [1]. In selected patient populations within recent clinical phase II trials, the m-OS ranges from 19 to 22 months, probably due to improvement in supportive care and more aggressive salvage therapy. Even with current standard of care (concomitant chemo-radiotherapy followed by adjuvant chemotherapy with temozolomide) for newly diagnosed GBM, the majority of patients recurs within a year. Available salvage therapies at recurrence are modestly effective, and no single treatment can be considered as the standard of care.
Angiogenesis has emerged as an attractive therapeutic target for therapy [2], and inhibition of vascular endothelial growth factor (VEGF) mediated signaling has recently received considerable attention in the targeting of recurrent malignant gliomas. Angiogenesis consists in the development of new blood vessels from preexisting vessels that occurs during embryonic and after birth to contribute to organ growth. High grade gliomas (HGG), especially GBM, are particularly vascularized and histologically characterized by neovascularization, and overexpression of VEGF [3, 4]. Bevacizumab, a humanized monoclonal antibody against circulating VEGF-A, has demonstrated promising radiological response rates (37.8–70.6 %) when used alone or in combination with irinotecan in recurrent GBM [5–7]. Other reported benefits are a decreased need for corticosteroids in 33–72.7 % of patients [3, 8, 9], and temporary improvement in neurological functions [5], although the survival data were less impressive with progression-free survival at 6 months (PFS-6) in 25–50 % [6, 10] of the patients. Bevacizumab is generally well tolerated with an acceptable safety profile, although rare and potentially life-threatening adverse events have been identified. The most common side effects are hypertension, fatigue, proteinuria, and poor wound healing [11], but also other potentially more serious adverse events such as venous and arterial thromboembolism, already not uncommon complications in patients with tumours, CNS and non-CNS haemorrhage, gastro-intestinal (GI) perforation, and reversible posterior leukoencephalopathy are reported.
We reviewed the available published data to investigate the relationship between the use of bevacizumab and VTE, CNS and non-CNS haemorrhage in patients with malignant gliomas, treated with or without concomitant anticoagulant therapy.
Materials and methods
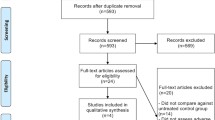
We reviewed the scientific literature to assess whether there was a significant increase in VTE and major bleeding events in patients with brain tumours receiving anti-VEGF therapies. A Medline research was performed including articles published since January 2005 up to July 2011. The search strategy combined terms for brain tumours/neoplasms and angiogenesis inhibitors to identify relevant information. Studies that have examined patients with malignant gliomas treated with either bevacizumab alone or combined with radio-chemotherapy, and with or without anticoagulant therapy, were considered for the analysis.
Two authors independently evaluated 476 records. Articles with population group and/or type of intervention not adequate to our endpoints (VTE, CNS and non-CNS haemorrhage), were eliminated. Twenty-seven full-text articles were assessed for eligibility, including retrospective analysis, retrospective reviews, and open-label trials. Only two studies deal with the use of bevacizumab and anticoagulants. We considered only articles reporting safety data.
Data were extracted independently by three authors using a pre-determined form. Quality was assessed using standardized criteria evaluating methodological quality (external validity, risk of bias according to Cochrane criteria, patient population features, outcome, follow-up, drop out, randomization, blinding). Data about type of antiangiogenic drug, incidence of venous/arterial thromboembolism, and of haemorrhage were extrapolated too. Disagreements on extractions were resolved by discussion.
We analysed the quality of included studies and the incidence of deep vein thrombosis (DVT)/pulmonary embolism (PE) and haemorrhage for antiangiogenic therapy alone, combined with other chemotherapy or with chemo- and radiotherapy.
We reported all VTE and haemorrhages evaluating the severity according to Common Terminology Criteria for Adverse Event (CTCAE) version 3.0. We defined as serious events any event of grade 3 or greater. Data have been shared to obtain the absolute number of VTE and bleedings in patients treated with bevacizumab alone, bevacizumab combined with chemotherapy, and bevacizumab used during concomitant chemo-radiotherapy.
To evaluate the association between treatment with bevacizumab alone or bevacizumab plus chemotherapy or bevacizumab plus chemo-radiotherapy and serious thromboembolic or bleeding events, we used the Chi-square test. We also evaluated the association between bevacizumab (alone or with other antineoplastic treatments) used with and without anticoagulants and serious bleeding events using the Chi-square test with Yates' correction.
Results
We identified 27 full-text articles reporting the use of bevacizumab in neuro-oncological patients. Bevacizumab alone was used in two studies [10, 14], combined with other drugs in 20 studies [3, 5–7, 12–28] in recurrent GBM, combined with radio-chemotherapy in four studies [29–32] in newly diagnosed GBM, and in one [33] in recurrent HGG. In two studies [12, 13], bevacizumab was associated with anticoagulants including warfarin, low molecular weight heparin (LMWH) and fondaparinux.
A total of 2,208 patients with brain tumours were analysed to investigate the relationship between the use of bevacizumab and VTE and bleedings.
VTE ≥grade 3 including DVT and PE was seen in 4.27 % of patients treated with bevacizumab alone. In patients treated with bevacizumab and concomitant chemotherapy, VTE was 4.19 %; while in patients treated with bevacizumab, radiotherapy and chemotherapy were 7.46 % (Table 1). Incidence of serious VTE between patients treated with bevacizumab alone, patients treated with bevacizumab plus chemotherapy, and patients treated with bevacizumab plus chemo-radiotherapy was not significantly different (p = 0.091).
The incidences of CNS and non-CNS haemorrhages greater than grade 3 in patients treated with bevacizumab alone were 0.4 %. In patients treated with antiangiogenic and chemotherapy, CNS and non-CNS haemorrhages were 0.84 and 0.97 %, respectively; the percentages of bleeding, both CNS and non-CNS, in patients treated with combination of bevacizumab, chemotherapy and radiotherapy were 0.74 and 1.11 %. While considering patients treated with bevacizumab and anticoagulant treatment associated, the percentages of CNS and non-CNS bleeding were 8.2 and 2.3 %, respectively (Table 2). Incidence of serious hemorrhagic events between patients treated with bevacizumab alone, patients treated with bevacizumab plus chemotherapy, and patients treated with bevacizumab plus chemo-radiotherapy was not significantly different (p = 0.307). Incidence of serious hemorrhagic events in patients treated with bevacizumab (alone or with other antineoplastic treatments) with anticoagulants was significantly increased compared to patients treated with bevacizumab without anticoagulants (p < 0.001).
Discussion
VTE represents one of the most important causes of morbidity (hospitalization, anticoagulation use, bleeding complications, increased risk of recurrent VTE, cancer treatment delays) [34] and mortality in cancer patients [35]. Overall, approximately 20 % of all VTE cases occur in patients with cancer [36] and the true extent of this complication may be underestimated [37].
The incidence of DVT and/or PE in patients with a brain tumour was found to be 120:100.000—the second highest rate for any malignancy [38] from Medicare Provider Analysis and Review Record. Both retrospective and prospective studies have suggested a particularly high incidence of VTE in patients with malignant gliomas [39, 40], from 2 to 60 %.
The mechanism of VTE development is multifactorial and neuro-oncological patients have many risk factors including histologic diagnosis of GBM (intraluminal thrombosis in the tumour pathological specimen), larger tumour size (high levels of procoagulant factors, use of high-dose steroids and more probability of motor deficit), presence of leg paresis (one of the most consistently identified factor due to the absence of the muscle pump effect with venous stagnation), older age (procoagulant factors increase with age, but anticoagulant proteins remain stable), more lengthy surgery (operative time more than 4 h), entity of surgery (subtotal resection versus total resection), chemotherapy (it reduces fibrinolytic activity), radiotherapy and steroids [41, 42].
Also, the novel antiangiogenic agents, as inhibitors of VEGF, seem to increase the risk of thromboembolic events and haemorrhage.
Because of the significant clinical improvement of antiangiogenic therapy in patients with recurrent HGG, the use of bevacizumab has increased in clinical practice despite its possible risks, but this possible adverse event should be considered, monitored and better defined.
The mechanism of anti-VEGF of antiangiogenic therapy may explain the development of VTE; it may expose subendothelial procoagulant phospholipids causing thrombosis by inhibition of VEGF-induced endothelial regeneration. Inhibition of VEGF may also predispose to VTE increasing haematocrit and thus blood viscosity through the surplus of erythropoietin. Moreover, bevacizumab, with its cytotoxic effect, can increase the release of procoagulant factor by tumour itself and the expression of cytokines which contribute to the development of thrombi [43, 44]. On the other hand, the mechanism of bleeding is not well known. VEGF is involved in endothelial cell survival and integrity of vascular system, and its inhibition could decrease the repair of damaged endothelial cells thus inhibiting the coagulation cascade regulated by tissue factor. Cases of thrombocytopenia in patients treated with bevacizumab and chemotherapy that could predispose to haemorrhage are described [45].
Our results seem to show that risk of VTE does not differ between patients treated with bevacizumab alone and patients treated with bevacizumab plus chemotherapy (4.27 vs 4.19 %), while it seem to increase when bevacizumab is associated with chemo-radiotherapy (7.46 %). This difference, even if not statistically significant, could be explained by the fact that patients undergoing concomitant treatment are at higher risk to develop thrombotic events because of the recent neurosurgery and the frequent concomitant steroid therapy in the perioperative setting and during radiotherapy.
If we consider all grades of toxicity (1–5 grade), the percentage increases up to 6.95 % for bevacizumab alone and 11.19 % when bevacizumab is associated with chemo-radiotherapy. However, the risk linked to antiangiogenic therapy, although administered alone, remains slightly high for patients with glioma.
It is important to underline that even if our data highlight a thromboembolic risk linked to the use of bevacizumab in addition to chemo-radiotherapy, an important phase III study conducted by Roche (AVAglio) [46], designed to evaluate the efficacy and safety of bevacizumab in combination with radio and chemotherapy, has recently completed the enrolment without showing an unacceptable safety profile. Arterial thromboembolic events greater than grade 3, were reported in 1.3 and 4.1 % in patients treated with RT + temozolomide + placebo and RT + temozolomide + bevacizumab, respectively. Venous thromboembolic events were reported in 8.1 and 7.3 % in patients treated with RT + temozolomide + placebo and RT + temozolomide + bevacizumab, respectively.
Regarding intracranial haemorrhages greater than grade 3, there are no significant differences between antiangiogenic therapy administered alone or in combination with radio-chemotherapy (0.6 vs 0.74 %); while regarding non-CNS bleeding (GI bleeding, epistaxis), data confirmed an increased risk in patients treated with bevacizumab and radio-chemotherapy (0.4 vs 1.11 %). Whereas, considering all grades of toxicity, CNS and non-CNS bleeding were more frequently seen in patients receiving bevacizumab associated with other chemotherapy (2.71 and 7.04 %, respectively).
Concomitant anticoagulant treatment seems to increase hemorrhagic risk of any grade, including severe bleeding. In fact, data show 8.2 % of CNS haemorrhage greater than grade 3 in bevacizumab plus anticoagulant; the percentage increases up to 14.1 % if we consider all grades of toxicity. On the other hand, regarding non-CNS haemorrhage, the percentage is around 2.3 % both for all toxicity and for only ≥3 grade.
Although bevacizumab is considered a safety therapy in terms of tolerability, and 50 % of treated patients can reduce or discontinue corticosteroids therapy with evident clinical benefit and improvement of quality of life, this anti-VEGF agent has a well-recognized complications that include hypertension, proteinuria, delay in wound healing, and leukoencephalopathy. Our data found also a not negligible risk under thromboembolic/hemorrhagic profile especially in neuro-oncological patients undergoing chemo-radiotherapy treatment. The hemorrhagic risk is likely to increase in those patients on anticoagulant treatment.
Conclusions
With the limitation of heterogeneity of the selected studies and the likelihood of bias, the analysis suggests that bevacizumab alone seems to be associated with a slightly high risk for developing a thromboembolic event. Bevacizumab combined with chemo- and radiotherapy seems to increase the risk to develop VTE and the use of anticoagulants combined with bevacizumab seems related to the increase in serious bleedings (both CNS and non-CNS). Actually, anti-VEGF plus anticoagulants data are based on only two studies; the serious lack of information about the safety of antiangiogenic treatment in association with anticoagulant therapy can be attributed to the few available clinical trials and also to the absence of guidelines on the prevention and management of these complications. Further studies on antiangiogenic toxicities are needed to understand the true risk/benefit ratio of therapy in HGG patients and also the relationship between dose received, duration of treatment and side effects.
References
Stupp R, Hegi ME, Mason WP et al (2009) European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Tuettenberg J, Friedel C, Vajkoczy P (2006) Angiogenesis in malignant glioma: a target for antitumor therapy? Crit Rev Oncol Hematol 59(3):181–193
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Kargiotis O, Rao JS, Kyritsis AP (2006) Mechanisms of angiogenesis in gliomas. J Neurooncol 78(3):281–293
Vredenburgh JJ, Desjardins A, Herndon JE 2nd et al (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13(4):1253–1259
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Zuniga RM, Torcuator R, Jain R et al (2009) Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol 91:329–336
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11(1):83–95
Vredenburgh JJ, Cloughesy T, Samant M et al (2010) Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist 15(12):1329–1334
Raizer JJ, Grimm S, Chamberlain MC et al (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116(22):5297–5305. doi:10.1002/cncr.25462
Armstrong TS, Wen PY, Gilbert MR et al (2012) Management of treatment-associated toxicities of anti-angiogenic therapy in patients with brain tumors. Neuro Oncol 14(10):1203–1214
Nghiemphu PL, Green RM, Pope WB et al (2008) Safety of anticoagulation use and bevacizumab in patients with glioma. Neuro Oncol 10(3):355–360
Norden AD, Bartolomeo J, Tanaka S et al (2011) Safety of concurrent bevacizumab therapy and anticoagulation in glioma patients. J Neurooncol 106(1):121–125
Chamberlain MC, Johnston S (2009) Bevacizumab for recurrent alkylator-refractory anaplastic oligodendroglioma. Cancer 115(8):1734–1743. doi:10.1002/cncr.24179
Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112:2267–2273
Kang TY, Jin T, Elinzano H, Peereboom D (2008) Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol 89:113–118
Socinski MA, Langer CJ, Huang JE et al (2009) Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol 27:5255–5261
Taillibert S, Vincent LA, Granger B et al (2009) Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology 72:1601–1606
Poulsen HS, Grunnet K, Sorensen M et al (2009) Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol 48(1):52–58
Thompson EM, Dosa E, Kraemer DF et al (2010) Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery 67(1):87–93
Hasselbalch B, Lassen U, Hansen S et al (2010) Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: a phase II trial. Neuro Oncol 12:508–516
Verhoeff JJ, Lavini C, van Linde ME et al (2010) Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 21:1723–1727
Scott BJ, Quant EC, McNamara MB et al (2010) Bevacizumab salvage therapy following progression in high-grade glioma patients treated with VEGF receptor tyrosine kinase inhibitors. Neuro Oncol 12(6):603–607
Sathornsumetee S, Desjardins A, Vredenburgh JJ et al (2010) Phase II trial of bevacizumab and erlotinib in patients with recurrent malignant glioma. Neuro Oncol 12:1300–1310
Francesconi AB, Dupre S, Matos M et al (2010) Carboplatin and etoposide combined with bevacizumab for the treatment of recurrent glioblastoma multiforme. J Clin Neurosci 17:970–974
Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, McLendon RE, Herndon JE 2nd, Coan A, Threatt S, Friedman AH, Friedman HS (2011) Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer 117(23):5351–5358. doi:10.1002/cncr.26188
Hofer S, Elandt K, Greil R et al (2011) Clinical outcome with bevacizumab in patients with recurrent high-grade glioma treated outside clinical trials. Acta Oncol 50:630–635
Fraum TJ, Kreisl TN, Sul J, Fine HA, Iwamoto FM (2011) Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neurooncol 105(2):281–289. doi:10.1007/s11060-011-0579-4
Lai A, Filka E, McGibbon B et al (2008) Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys 71(5):1372–1380
Vredenburgh JJ, Desjardins A, Kirkpatrick JP, Reardon DA, Peters KB, Herndon JE 2nd, Marcello J, Bailey L, Threatt S, Sampson J, Friedman A, Friedman HS (2012) Addition of Bevacizumab to Standard Radiation Therapy and Daily Temozolomide Is Associated with Minimal Toxicity in Newly Diagnosed Glioblastoma Multiforme. Int J Radiat Oncol Biol Phys 82(1):58–66. doi:10.1016/j.ijrobp.2010.08.058
Lai A, Tran A, Nghiemphu PL et al (2011) Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 29:142–148
Vredenburgh JJ, Desjardins A, Reardon DA et al (2011) The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res 17:4119–4124
Niyazi M, Ganswindt U, Schwarz SB et al (2010) Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys 82(1):67–76
Khorana AA, Connolly GC (2009) OncolAssessing risk of venous thromboembolism in the patient with cancer. J Clin 27(29):4839–4847
Chew HK, Wun T, Harvey D et al (2006) Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 166(4):458–464
Lee AY (2005) Deep vein thrombosis and cancer: survival, recurrence, and anticoagulant choices. Dis Mon 51(2–3):150–157
Gao S, Escalante C (2004) Venous thromboembolism and malignancy. Expert Rev Anticancer Ther 4(2):303–320
Levitan N, Dowlati A, Remick SC et al (1999) Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy. Risk analysis using medicare claims data. Medicine (Baltimore) 78(5):285–291
Constantini S, Kornowski R, Pomeranz S, Rappaport ZH (1991) Thromboembolic phenomena in neurosurgical patients operated upon for primary and metastatic brain tumors. Acta Neurochir (Wien) 109(3–4):93–97
Sawaya R, Zuccarello M, Elkalliny M, Nishiyama H (1992) Postoperative venous thromboembolism and brain tumors: part I. Clinical profile. J Neurooncol 14(2):119–125
Marras LC, Geerts WH, Perry JR (2000) The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence-based review. Cancer 89(3):640–646
Semrad TJ, O'Donnell R, Wun T et al (2007) Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 106(4):601–608
Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300(19):2277–2285
Kilickap S, Abali H, Celik I (2003) Bevacizumab, bleeding, thrombosis, and warfarin. J Clin Oncol 21(18):3542
Hapani S, Sher A, Chu D, Wu S (2010) Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta-analysis. Oncology 79(1–2):27–38
Chinot OL, de La Motte Rouge T, Moore N et al (2011) AVAglio: phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther 28(4):334–340
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simonetti, G., Trevisan, E., Silvani, A. et al. Safety of bevacizumab in patients with malignant gliomas: a systematic review. Neurol Sci 35, 83–89 (2014). https://doi.org/10.1007/s10072-013-1583-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1583-6