Abstract
Spinal cord injury (SCI) is a disease that affects millions of people worldwide, causing a temporary or permanent impairment of neuromotor functions. Mostly associated to traumatic lesions, but also to other forms of disease, the appropriate treatment is still unsure. In this review, several ongoing studies are presented that aim to provide methods of prevention that ensure quality of life, and rehabilitation trends to patients who suffer from this injury. Stem cell research, highlighted in this review, seeks to reduce damage caused to the tissue, as also provide spinal cord regeneration through the application of several types of stem cells. On the other hand, research using brain–computer interface (BCI) technology proposes the development of interfaces based on the interaction of neural networks with artificial tools to restore motor control and full mobility of the injured area. PubMed, MEDLINE and SciELO data basis analyses were performed to identify studies published from 2000 to date, which describe the link between SCI with stem cells and BCI technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the current concerns in the field of neuroscience is the injury repair/neuroregeneration process. Since this is a form of neuroplasticity, one of the goals is to promote or reinstall functionality in the injured area [1]. Particularly, spinal cord injury (SCI) has been the focus of extensive research in neuroscience; this is characterized by damage in the spinal canal, resulting in a temporary or permanent impairment of neuromotor functions [2]. This field in particular gave rise to various hypotheses that attempt to explain the control of motor actions. The SCI may result from traumatic injuries or other congenital, degenerative, neoplastic and infectious processes, neurological and vascular disorders. Recent studies indicate that trauma is the leading cause of SCI among young people aged between 15 and 40 years. Motor vehicle accidents are the main cause, followed by firearm injury, sport accidents and direct trauma [3]. After injury, evaluations should be performed at various levels to better intervene in the subsequent recovery processes. The American Spinal Cord Injury Association (ASIA) devised a qualitative scale for evaluation and classification of neurological deficit caused after injury. This assessment classifies a SCI into complete and incomplete, and evaluates the extent of damage to the spinal cord tissue, firstly verifying if the primary functions are preserved [4].
The histological, biochemical and functional aspects of the disease have attracted great interest among researchers, who seek to improve the quality of life of patients suffering from this disabling injury [5]. Different treatments have been employed aiming to contain the structure involvement and injury severity. These treatments are based on studies of spinal cord regeneration using neurotrophic factors, fetal bone marrow graft, peripheral nerve graft, antibodies against myelin-associated proteins and stem cells [6]. Concomitantly to these treatments, the emergent field of neuroengineering has made substantial discoveries in brain research and brain-computer interfacing (BCI), enabling patients who suffered severe body paralysis to find other ways to restore full mobility of the injured area [7].
With this in mind, the present paper reviews the types of SCI, the basic concepts of stem cells and the BCIs, and presents the clinical applications of stem cells and BCI on motor control restoration of patients with spinal cord lesions, to create an elegant proposal regarding the clinical applications of these potential therapies in the upcoming years.
Spinal cord lesions: basic concepts
This study will mainly emphasize injuries that affect the conduction of sensory-motor impulses in the spinal cord promoting loss of sensation and movement. Trauma in the spinal cord region undertake the tract and fascicles, which comprise ascendant and descendent pathways responsible for transmitting the information to all structure of the central nervous system (CNS) and the peripheral nervous system (PNS). Damage in the spinal cord can be mainly attributed to trauma, but also may result from other etiologies. Lesion intensity can range from mild to more severe injuries, and may be complete or incomplete; in the last the sensory-motor function in the region that was not affected is still preserved. The spinal cord hemisection, also known as Brown-Sequard Syndrome, affects the corticospinal tract (causing ipsilateral loss of motor function), the fasciculus gracilis and the cuneate nucleus (causing loss of conscious proprioception and discriminative touch) [8]. In addition, spinothalamic tract (anterior and lateral) dysfunction causes contralateral thermal and pain sensitivity, and loss of light touch [9, 10]. In complete lesions, the motor function is totally interrupted below the level of trauma. Sensory and autonomic motor tracts are disrupted, causing nerve root damage in the segmented section. A complete transverse section of the spinal cord depending on the level of the lesion can affect several body parts [11].
The damage caused by injury is analyzed through neurological assessments based on the sensory maps and key muscles. The ASIA Impairment Scale (AIS) aims to define exactly the level of neurological injury and the degree of functional impairment, aiming a prognostic classification [12]. The scale evaluates the sensitivity levels from C2 through S4–S5, and the function of muscle groups and reflexes related to the nerve roots from C5 to T1 (brachial plexus) and from L2 to S1 (lumbar plexus) [4]. The lesions are classified into five levels (A–E); A = Complete. No sensory or motor function is preserved in the sacral segments S4–S5; B = Sensory Incomplete. Sensory but not motor function is preserved below the neurological level and includes the sacral segments S4–S5 [light touch, pin prick at S4–S5: or deep anal pressure (DAP)], and no motor function is preserved more than three levels below the motor level on either side of the body; C = Motor Incomplete. Motor function is preserved below the neurological level, and more than half of key muscle functions below the single neurological level of injury (NLI) have a muscle grade <3 (grades 0–2); D = Motor Incomplete. Motor function is preserved below the neurological level, and at least half (half or more) of key muscle functions below the NLI have a muscle grade ≥3; E = Normal. If sensation and motor function as tested with the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) are graded as normal in all segments, and the patient had no prior deficits, then the AIS grade is E. Someone without an initial SCI does not receive an AIS grade.
Stem cells: types and functions
The potential uses of stem cells as a medical treatment have attracted extensive research interest in the recent years. Stem cells are unspecialized cells that have the unique ability of self-renewal and to differentiate into multiple specific cell types, in response to a particular signal [13]. These cells are divided into two classes, embryonic and adult stem cells, according to their origin. Embryonic cells are subdivided into totipotent and pluripotent cells, according to their potential ability to differentiate into specialized tissue. The totipotent type is able to differentiate into all cell types in an adult body [14]; pluripotent cells have the potential to differentiate into specialized ectoderm, mesoderm or endoderm cells [15]. However, despite these unique properties, the use of embryonic stem cells for rehabilitation treatments is still polemic, since it involves ethnic and religious questions. Though, in some countries researches using in vitro embryo discarded in fertilization process are permitted [14].
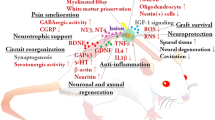
In majority, adult stem cells are denominated multipotent cells capable to originate only a few types of cells lineages [16], but being a non-embryonic potential source have ethnic and religious advantage to neuroscience research [17]. These cells are accessible in several organs and tissues of human body, and in extra-embryonic structures such as the umbilical cord and placenta [18]. There are also the mesenchymal stem cells (MSCs), predecessors of the conjunctive and muscular tissue cells, which are found in the umbilical cord [3]. Another extraction source of the MSCs is found in the bone marrow, on the estromal region [19]. The cells of this region are able to form mesodermal tissue such as cells from the bone, cartilaginous, adipose and muscular tissue [20]. Furthermore, in appropriate fertilization conditions and provided specific stimulus, MSCs can also differ in central and peripheral nervous tissue [21]. Therapies with these cells are increasing due to its easy availability and capacity to propagate in culture; apart from being non-immunogenic, which eliminates the problems of rejection and permits an autologous transplant [22]. Another source of multipotent stem cells with the capacity of differentiation in nervous tissue is found in the olfactory epithelium [11]. Denominated neural stem cells (NSCs) are found in an accessible region of the brain that may be removed from the patient without causing significant damage [23]. When they grow properly, they are capable to secrete several growth factors such as NGF (neural growth factor), BDNF (brain-derived neurotrophic factor), GDNF (glial cell line-derived neurotrophic factor), FGF (fibroblast growth factor), and VEGF (vascular endothelial growth factor), that are known to play a role in the neurogenesis, promoting the growth, maintenance and survival of the nervous cells. Due to its characteristics, the olfactory cells have an important supporting function in axonal regeneration, and they have been used in diverse research on nervous tissue regeneration [3].
The procedures with stem cells in the field of neuroscience bring an exciting prospect in the treatment of thousands of neurodegenerative diseases and damaged tissues. Due to differentiation and self-regeneration capacities, regenerative therapies using stem cells are able to provide functional improvements to the individual.
Stem cells applied to regeneration of neural tissue in spinal cord lesions
In the search for efficient treatments, the use of several sources of stem cells, in group or alone, is researched and reported in experiments with injured animals or human beings [15]. As micro-environment conditions of rodent models appear to be similar to that found in humans, several techniques are being employed in these populations. However, these techniques should be applied in humans only after proving the efficiency and acceptance of the therapy by the ethics committee [24]. In the following, some treatment will be presented showing the functional gains in animals and humans with SCI. Investigations of SCI in rodent models emphasize that the use of Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell to remyelinate axons, reduces tissue damage [25] and helps to prevent the return of chronic pain [26], improving the locomotor function [27, 28]. At present, one of the most important studies that uses embryonic stem cells as a therapy for SCI regeneration, is developed by Geron Corporation. From embryos discarded in the process of fecundation in vitro, Oligodendrocytes (GRNOPC1) were extracted and employed in therapies with SCI rodent models [16]. Seven days after SCI, the GRNOPC1 were implanted in the lesion site. With this procedure, it was possible to note the production of neurotrophic factor and remyelination of axons, causing significant repair in locomotor capacity and body support. In January 2009, the corporation received permission of Food and Drug Administration (FDA) to start the first clinical essay in humans with acute SCI, classified degree A in the evaluation of ASIA. The first stage of the research aimed to evaluate the security and tolerability of the procedure applied in humans [29].
Other research line constituted by studies with non-embryonic stem cells sources, present methods considered safer and more viable, considering ethical and religious aspects [30]. Studies with insertion of NSCs in rodents showed axonal regeneration [31], and recovery of speed conduction and locomotor function [32]. In another study, patients with chronic and stable SCI at degree A of ASIA, had NSCs of mucous olfactory membrane inserted [33]. Improvement in ASIA scores [34], and returning of sensibility and voluntary contraction of the anal sphincter [35] were observed in the transplanted individuals. Trials using MSCs in damaged animals permitted alterations in the site of the injury; acute and less acute effects on the tissue were reduced promoting a permissive site for remyelination and local repair [19]. Functional regeneration and gradual improvement of locomotor function as assessed by BBB score (Basso-Beattie-Bresnahan) [36], incidences of spontaneous movements, and neural growth have also been evidential after MSCs graft in rodents [20, 37]. Approaches with combined sources of non-embryonic stem cells are also applied in regenerative therapies. Moviglia et al. [38] have reported skin sensibility in patients with SCI after combined applications of MSCs and NSCs. Moreover, the application of diverse sources of NSCs was capable to promote urologic, sexual and intestinal function repairing [18]. Although the main objective of the studies with stem cells is the search for methods to restore the neural circuits, the therapies developed until the present moment still do not show an adequate mechanism to completely restore the damage in the spinal cord tissue.
Brain–computer interface (BCI): future challenges
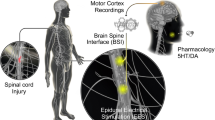
During the last decade, the neuroscience research has been progressing in equal to technological improvement. A considerable progress has been made in understanding the CNS organization and, mainly, how the brain is capable to codify and manipulate high quantity of complex information [39–42]. The neuroprosthetic devices based on brain–computer interface (BCI) technology are a promise for body mobility regeneration in individuals who suffered any degenerative injury, limbs loss and neurological diseases [43]. Interface based on non-invasive techniques are applied in several clinical researches to detect and evaluate modular activities in the brain which are related to sensorial inputs, intention and cognitive states [44–47]. Besides, noninvasive mechanisms have been developed to help disabled individuals in daily living tasks. These types of BCI are composed of systems for answering “yes” or “no” to questions, managing the user’s environment, accessing the internet, or controlling a hand orthosis. Thus, disabled individuals can perform basic daily living tasks, such as word processing, catching an object, sending e-mails, or operating a motorized wheelchair [48].
On the other hand, invasive techniques have been developed to establish the direct motor control domain [49]. Due to the disconnection between the brain and the spinal cord the information provided on the motor cortex is not transmitted through the pyramidal tract to the effector organ [50, 51]. These techniques allow a direct channel of communication between the CNS and the prosthetic devices, providing a way to restore the lost capacity in spinal cord injured individuals [52, 53]. Applying the BCIs, commands can be extracted directly from cerebral activities. The multiple channels of neural signals are recorded simultaneously and maintained in a decodification module. The interface will process and encode the neural signals in real time, extracting motor commands and sending to a robotic limb (neuroprosthesis) the brain motor intention, which still reproduces a motor thought [54].
The interaction of the proprioceptive system with the motor intention is vital to perform a precise and natural movement [7]. The proprioceptive feedback rapidly corrects the movement [55]. Thus, the association with the proprioceptive and the visual feedback allows a better control of the device [56]. Lebedev and Nicolelis [49] emphasize that the BCI should promote to the limbs the same performance, sensation and action of the real limb, otherwise it can be rejected by the individual. The neuroscientists also emphasize that to perform well with the BCI, multiple artificial signals coming from pressure and position sensors inserted in the prosthetic limb should be assimilated to the cerebral representation. These feedback signals are capable to educate the brain to incorporate the artificial proprieties of the neuroprosthesis, modify neurons located in cortical and subcortical areas that maintain the representation of the patient’s body. The future importance of such BCI applications will depend on their capacities, practicality, reliability and acceptance by particular groups of users, and on the extent to which they have substantial advantages over conventional assistive technology. Therefore, we should evaluate what has actually been achieved in patients with spinal cord lesions thus far.
Clinical applications of BCI on motor control restoration in patients with spinal cord lesions
The clinical applications via noninvasive and invasive BCI may serve as evidence for the feasibility of controlling neuroprosthesis, providing a solid basis for the development of ‘thought’-controlled neuroprosthesis, which seem to assist patients with severe paralysis to motor control restoration. Pfurtscheller et al. [57] investigated the possibility of modulating sensorimotor rhythms in a young tetraplegic subject since 1998. The patient was able to modulate sensorimotor rhythms (i.e., central mu-rhythms) after extensive training, controlling an electro stimulation device (FES) applied to the forehand muscles. Thus, he was able to grasp a glass and bring it to his mouth after 4 months of regular training. Three years ago, Pfurtscheller et al. [58] examined two tetraplegic patients (male, SCI at level C5 and sub-C5, respectively) using the same task. FES restores the grasp function of the left hand of the first patient using surface electrodes applied to forearm muscles. The patient learned to induce his sensorimotor rhythms (i.e., ERD—17 Hz oscillations) through 4 months of foot MI training. With respect to the second patient, a FES system was implanted in his right hand and arm. After 3 days of feedback training, the patient learned to induce an ERD pattern of sensorimotor rhythms during left-hand MI, allowing a binary control signal to emulate the shoulder joystick used to operate the FES system.
Trials with invasive methods have been made in animals and humans; however, the experiments still need to overcome several obstacles. One of those refers to neural recording that lacks quality and stability, to prevent inflammatory reaction and cerebral tissue damage. In addition, the degree of freedom in interfaces needs to be enhanced to provide a stable performance of the neuroproteases. In relation to the amount of neurons that will efficiently control this device, Nicolelis and Lebedev [59] includes in his research a fundamental question that is still being studied: “What should be the satisfactory amount of neurons to activate the motor control restoration?” To answer this question, studies are being conducted in monkeys at the University of Duke in North Carolina. The neuroproteases, implanted in motor areas, are based on bidirectional systems in which the motor control signals are extracted from the brain using a group of multi-electrodes. The sensorial feedback made by tactile, and proprioceptive stimuli and other functional signals are sent to the sensorial areas of the brain through intracortical stimulation (ICMS) [60]. Based on these methods, The Walk Again Project (WAP), an international scientific consortium led by researchers of Duke University and composed by two headquarters,Footnote 1 has been trying to develop and incorporate the first brain–machine interface capable of restoring the corporal mobility in SCI or neurodegenerative diseases’ patients. This interface called “wearable robot” will make possible that movements from all parts of the patient’s body be controlled and fulfilled by his voluntary brain activity. This exoskeleton will be designed to sustain and transport the patient’s body according to his mental will [51].
Another type of BCI, the neuromotor prostheses, referred by Hochberg and co-workers [61], is capable to guide the movements through the patterns of neural activities in the primary motor area (M1). The authors have shown that the neuroprosthesis requires a detection sensor of the multiple neuron activities and an encoder to translate a group of discrete patterns in motor commands. In order to make these commands occur correctly, a neuroprosthesis should have a device capable to involve the effectors in the determinate action. In 2004, Hochberg et al. [61] made the first trial to implant the BCI. Micro-electrodes have been implanted in M1 area in a 25-year-old woman that in 2001 suffered a SCI at the cervical level resulting in tetraplegia. After a functional magnetic resonance imaging (fMRI) it could be observed that action potentials were still being generated in the cortical area, even after 3 years of SCI. In the same year, a second patient was included in the research, a 55-year-old man that suffered a SCI in 1999, in C4 level. After 7 months of adaptation to micro-electrodes implement in the M1 area, the recording confirmed neural activity in the M1 area.
In order to stabilize the transformation of the firing patterns in the M1, Hochberg et al. [61] mentioned that neural filters generators of bidimensional output signals to the computer control position should be created with a new approach for people with paralysis. In this study, the filters have been used to decode the activity and direction of the cursor by neural activation. The desired action in the first moment consisted in the mental visualization of the hands controlling a computer mouse. After that, the subject was instructed to follow the movement of the cursor in the computer. The results of the study indicate that even after years of damage, and in absence of sensorial feedback and limb movements, the neurons of the M1 area were still active and capable to decode the information related to a specific task. Therefore, the control signal of neural activity can be used in several interfaces. Simple assistance devices such as visual interface of e-mails and control of electronic equipment will help the injured individuals in basic tasks. Besides, Hochberg et al. [61] describe the employment of a prosthetic hand to the neural output. After the coupling, the subject imagined and produced verbal commands (open/close) leading the hand to open and close the fingers. Due to interfering factors such as the modest implanted area, the chosen approach for filters construction, and the attention and motion state, the subject showed an inferior level of control of actions when compared to healthy individuals.
Conclusion
As outlined in the previous sections two distinct methods were shown which can be considered promising approaches in neurorehabilitation for SCI progress; however, their true potential in the spinal cord repair have not yet clearly been shown. The use of stem cells in the therapeutic field is still dealing with barriers of ethics and religion. which do not allow researching with all types of stem cells. At the same time, the BCI technology is facing limitations in developing artificial tools which are capable of linking the living brain tissue and being incorporated. Then there is still no clear evidence that either stem cells or BCI technology presently makes the cure for SCI possible. Studies have been developed around the world, and maybe we will see one of these therapies being successful in the near future.
Notes
The Center for Neuroprosthetics at the École Polytechnique Fédérale de Lausanne (EPFL), in Switzerland, and the Laboratory of Dr. Gordon Cheng at the Technical University of Munich in Germany are the houses of the European headquarters of the WAP and the Latin American headquarters, the Brazilian National Institute of Brain–Machine Interface located in Natal, Brazil [51].
References
Bazley FA, All AH, Thakor NV, Maybhate A (2011) Plasticity associated changes in cortical somatosensory evoked potentials following spinal cord injury in rats. Conf Proc IEEE Eng Med Biol Soc 2011:2005–2008
Musienko P, Heutschi J, Friedli L, Van den Brand R, Courtine G (2012) Multi-system neurorehabilitative strategies to restore motor functions following severe spinal cord injury. Exp Neurol 235(1):100–109
Sobani ZA, Quadri SA, Enam SA (2010) Stem cells for spinal cord regeneration: current status. Surg Neurol Int 1:93
Gaspar MIFAS, Cliquet Junior A, Lima VMF, Abreu DCC (2008) Evaluation of ASIA and somatosensory evoked potential in individuals with paraplegia. Coluna 7(3):223–229
Barros Filho TEP, Oliveira RP, Tsanaclis AM, Barros EMK, Cristante AF, Palma RM et al (2002) An experimental model for the transplantation of fetal central nervous system cells to the injured spinal cord in rats. Rev Hosp Clín Fac Med S Paulo 57(6):257–264
Narazaki DK, de Barros Filho TEP, de Oliveira CRGCM, Cristante AF, Iutaka AS, Marcon R, Oliveira RP (2006) Spinal Cord regeneration: the action of neurotrophin-3 in spinal cord injury in rats. Clinics 61(5):453–460
Suminski AJ, Tkach DC, Hatsopoulos NG (2009) Exploiting multiple sensory modalities in brain–machine interfaces. Neural Netw 22:1224–1234
Filli LB, Weinmann O, Schwab ME (2011) Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown-Sequard syndrome. Brain 134(8):2261–2273
Choi Kyeong Bo, Lee Choon Dae, Chung Dai-Jin, Lee Sang-Ho (2009) Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc 46(5):505–510
Ghosh A, Sydekum E, Florent Haiss F, Peduzzi S, Zörner B, Schneider R, Baltes C, Rudin M et al (2009) Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci 29(39):12210–12219
Thuret S, Moon LDF, Gage FH (2006) Therapeutic interventions after spinal cord injury. Nat Rev Neurosci 7(8):628–643
Neves MA, Mello MP, Antonioli R, Freitas MRG (2007) Functional and clinical scales in management of individuals with traumatic injuries of spinal cord. Rev Neurosci 15(3):234–239
Bydlowski SP, Debes AA, Duarte SA, Janz FL, Cavaglieri RC, Maselli LMF (2009) Células-tronco do líquido amniótico. Ver Brás Hematol Hemoter 31 suppl 11 (in Portuguese)
Tewarie RSN, Hurtado A, Bartels RH, Grotenhuis A, Oudega M (2009) Stem cell-based therapies for spinal cord injury. J Spinal Cord Med 32(2):105–114
Rossi SL, Keirstead HS (2009) Stem cells and spinal cord regeneration. Curr Opin Biotech 20:552–562
Jones R, Lebkowski J, McNiece I (2010) Stem Cells. Biol Blood Marrow Transplant 16(1 suppl):S115–S118
Bydlowski SP, Debes AA, Maselli LMF, Janz FL (2009) Características biológicas das células-tronco mesenquimais. Rev Bras Hematol Hemoter 31(1 suppl):25–35
Ichim TE, Solano F, Lara F, Paris E, Ugalde F, Rodriguez JP et al (2010) Feasibility of combination allogeneic stem cell therapy for spinal cord injury: a case report. Int Arch Med 11(3):30
Wright KT, Masri WE, Osman A, Chowdhury J, Johnson WEB (2011) Concise review: bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells 29:169–178
Wu S, Suzuki Y, Ejiri Y, Noda T, Bai H, Kitada M et al (2003) Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J Neurosci Res 72:343–351
Abdallah BM, Kassem M (2008) Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther 15(2):109–116
Zietlow R, Lane EL, Dunnett SB, Rosser AE (2008) Human stem cells for CNS repair. Cell Tissue Res 331:301–322
Gögel S, Gubernator M, Minger SL (2011) Progress and prospects: stem cells and neurological diseases. Gene Ther 18:1–6
Liverman CT, Altevogt M, Joy JE, Johnson RT (2005) Spinal cord injury: progress, promise, and priorities. The National Academies Press, Washington
Zhang YW, Denham J, Thies RS (2000) Oligodendrocyte progenitor cells derived from human embryonic stem cells express neurotrophic factors. Stem Cells Dev 15:943–952
Glazova M, Pak ES, Moretto J, Hollis S, Brewer KL, Murashov AK (2009) Pre-differentiated embryonic stem cells promote neuronal regeneration by cross-coupling of BDNF and IL-6 signaling pathways in the host tissue. J Neurotrauma 26(7):1029–1042
Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K et al (2005) Human embryonic cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal injury. J Neurosci 25:4694–4705
Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead H (2005) Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49:385–396
Priest C, Davies A, Wirth E, Conta A, Polonskaya Y, Polonskowski J (2009) Preclinical development of oligodendrocyte progenitor cells derived from human embryonic stem cells for the treatment of spinal cord injury. Cell Transplant 18:231
Rabinovich SS, Seledtsov VI, Poveschenko OV, Senuykov VV, Taraban VY, Yarochno VI et al (2003) Transplantation treatment of spinal cord injury patients. Biomed Pharmacother 57(9):428–433
Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K et al (2007) Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 4(2):e39
Salazar DL, Uchida N, Hamers FPT, Cummings BJ, Anderson AJ (2010) Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE 5(8):e12272
Féron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S et al (2005) Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain 128:2951–2960
Huang H, Chen L, Wang H, Xiu B, Li B, Wang R et al (2003) Influence of patients’ age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chin Med J 116(10):1488–1491
Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G et al (2010) Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair 24:10–22
Cizková D, Rosocha J, Vanicky I, Jergova S, Cizek M (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26:1165–1178
Ankeny DP, McTigue DM, Jakeman LB (2004) Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol 190:17–31
Moviglia GA, Viña RF, Brizuela JA, Saslavsky J, Vrsalovic F, Varela G et al (2006) Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 8(3):202–209
Lebedev MA, Tate AJ, Hanson TL, Li Z, O’Doherty JE, Winans JA et al (2011) Future developments in brain–machine interface research. Clinics 66(S1):25–32
JdR Millán, Rupp R, Müller-Putz GR, Murray-Smith R, Giugliemma C, Tangermann M et al (2010) Combining brain–computer interfaces and assistive technologies: state-of-the-art and challenges. Front Neurosci. 4:161
Birbaumer N, Cohen LG (2007) Brain–computer interfaces: communication and restoration of movement in paralysis. J Physiol 579(3):621–636
Partil PG, Turner DA (2008) The development of brain–machine interface neuroprosthetic devices. Neurotherapeutic 5(1):137–146
Fagg AH, Hatsopoulos NG, de Lafuente V, Moxon KA, Nemati S, Rebesco JM et al (2007) Biomimetic brain machine interfaces for the control of movement. J Neurosci 27(44):11842–11846
Krepki R, Blankertz B, Curio G, Müller KR (2007) The Berlin Brain-Computer Interface (BBCI)—towards a new communication channel for online control in gaming applications. Multimed Tools Appl
Kreilinger A, Kaiser V, Breitwieser C, Williamson J, Neuper C, Müller-Putz GR (2011) Switching between manual control and brain–computer interface using long term and short term quality measures. Front Neurosci 5:147
Liao LD, Chen CY, Wang IJ, Chen SF, Li SY, Chen Bw et al (2012) Gaming control using a wearable and wireless EEG-based brain–computer interface device with novel dry foam-based sensors. J Neuroeng Rehabil 28(9):5
Doud AJ, Lucas JP, Pisansky MT, He B (2011) Continuous three-dimensional control of a virtual helicopter using a motor imagery based brain–computer interface. PLoS ONE 6(10):e26322
Andersen RA, Musallam S, Pesaran B (2004) Selecting the signals for a brain–machine interface. Curr Opin Neurobiol 14:720–726
Lebedev MA, Nicolelis MAL (2006) Brain–machine interfaces: past, present and future. Trends Neurosci 29(9):536–546
Friehs GM, Zerris VA, Ojakangas CL, Fellows MR, Donoghue JP (2004) Brain–machine and brain–computer Interfaces. Stroke. 35[suppl I]:2702–2705
Nicolelis M (2011) Muito além do nosso eu: a nossa neurociência que une cérebros e maquinas—e como ela pode mudar nossas vidas. São Paulo, Companhia das Letras (in portuguese)
Mahmoudi B, Sanchez JC (2011) A symbiotic brain–machine interface through value-based decision making. PLoS ONE 6(3):e14760
Scherberger H (2009) Neural control of motor prostheses. Curr Opin Neurobiol 19:1–5
Wang W, Collinger JL, Perez MA, Tyler-Kabara EC, Cohen LG, Birbaumer N et al (2010) Neural interface technology for rehabilitation: exploiting and promoting neuroplasticity. Phys Med Rehabil Clin N Am 21:157–178
Hatsopoulos NG, Donoghue JP (2009) The science of neural interface system. Annu Rev Neurosci 32:249–266
Suminski AJ, Tkach DC, Fagg AH, Hatsopoulos NG (2010) Incorporating feedback from multiple sensory modalities enhances brain–machine interface control. J Neurosci 30(50):16777–16787
Pfurtscheller G, Guger C, Müller G, Krausz G, Neuper C (2000) Brain oscillations control hand orthosis in a tetraplegic. Neurosci Lett 292:211–214
Pfurtscheller G, Müller GR, Pfurtscheller J, Gerner HJ, Rupp R (2003) Thought-control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett 351:33–36
Nicolelis MA, Lebedev MA (2009) Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nat Rev Neurosci 10:530–540
O’Doherty JE, Lebedev MA, Hanson TL, Fitzsimmons NA, Nicolelis MA (2009) A brain–machine interface instructed by direct intracortical microstimulation. Front Integr Neurosci 3:20
Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP (2006) Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442:164–171
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gongora, M., Peressutti, C., Machado, S. et al. Progress and prospects in neurorehabilitation: clinical applications of stem cells and brain–computer interface for spinal cord lesions. Neurol Sci 34, 427–433 (2013). https://doi.org/10.1007/s10072-012-1232-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-1232-5